Chemistry 260: Analytical Chemistry
advertisement

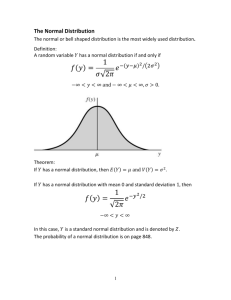
University of San Francisco Chemistry 260: Analytical Chemistry Dr. Victor Lau Room 413, Harney Hall, USF lauv@ace.usfca.edu What is Analytical Chemistry Using chemistry principle on analyzing something “unknown”…. Qualitative Analysis: the process of identifying what is in a sample Quantitative Analysis: the process of measuring how much of the substance is in a sample. Here is the SAMPLE … DO NOTHING ON THE RECEIVED SAMPLE !!!!!!!!!! LOOK AT THE SAMPLE Report all your observations on the log book before doing any non-destructive or destructive analysis Reading a Burette 1 The diagram shows a portion of a burette. What is the meniscus reading in milliliters? A. 24.25 B. 24.00 C. 25.00 D. 25.50 reference: http://www.sfu.ca/chemistry/chem110111/Lab/titration.html Reading a Burette 2 How about this is? A. 41.00 B. 41.10 C. 41.16 D. 41.20 Reading a Burette A 50 mL burette can be read to ± 0.01 ml, and the last digit is estimate by visual inspection. However, in order to be able to interpolate to the last digit, the perpendicular line of sight must be followed with meticulous care. Note in these two photographs, one in which the line of sight is slightly upward and the other in which it is downward, that an interpolation is difficult because the calibration lines don't appear to be parallel. upward downward perpendicular Section I: Math Toolkit I: Significant Figures Significant Figures is the minimum number of digits needed to write a given value in scientific notation without the loss of accuracy. To be simple, sig. figs = meaningful digits 9.25 x 104 9.250 x 104 9.2500 x 104 3 sig. figs. 4 sig. figs 5 sig. figs Significant Figures in Arithmetic Addition and Subtraction If the numbers to be added or subtracted have equal numbers of digits, the answer goes to the same decimal place as in any of the individual numbers. e.g. 18.998 403 2 (F) 18.998 403 2 (F) 83.80 (Kr) 121.796806 4 (KrF2) not significant Significant Figures in Arithmetic Multiplication and Division In multiplication and division, we are normally limited to the number of digits contained in the number with the fewest significant figures. e.g. 3.26 x 10 -5 1.78 5.80 x 10 -5 34.60 2.462 87 14.05 Significant Figures in Arithmetic Logarithms and Antilogarithms log y = x, means y = 10x A logarithm is composed of a characteristic and a mantissa log 339 = 2 .530 characteristic mantissa # of digits in the mantissa = # of sig. fig in the original number log 1,237 = 3.0924 Types of Error Every measurement has some uncertainty, which is called Experimental Error Experimental Error can be classified as Systematic, Random; and Gross Error Experimental Error Systematic Error Random Error Gross Error Consistent tendency of device to read higher or lower than true value “noise” Due to mistake e.g. uncalibrated buret Unpredicted Higher and lower than true value Precision and Accuracy Precision is a measure of the reproducibility or a result Accuracy refers to how close a measured value is to the “true “ value Absolute and Relative Uncertainty absolute uncertaint y Relative uncertaint y magnitude of measuremen t 0.02ml e.g. 0.002 12.35ml Percentage uncertaint y 100 relative uncertaint y e.g. 100 0.002 0.2% Propagation of Uncertainty When we used measured values in a calculation, we have to consider the rules for translating the uncertainty in the initial value into an uncertainty in the calculated value. A simple example of this is the subtraction for two buret readings to obtain a volume delivered Addition and Subtraction 1.76 (0.03) e1 1.89 (0.02) e2 - 0.59 (0.02) e3 3.06 ( e4) e1, e2, and e3 is the uncertainty of the measurements, respectively. e4 is the total uncertainty after addition/subtraction manipulation where as, e4 e1 2 e2 2 e3 2 that is, e4 (0.03) 2 (0.02) 2 0.02) 2 0.041 0.041 Although there is only one significant figure in the uncertainty, we wrote it initially as 0.041, with the first insignificant figure subscripted. Therefore, percentage of uncertainty = 0.041/3.06 x 100% = 1.3% = 1.3% 3.06 (+/- 0.04) (absolute uncertainty), or 3.06 (+/- 1%) Multiplication and Division For multiplica tion and division, first convert all uncertaint ies to percent relative uncertaint ies. Then calculate the error or the product or quotient as follows : %e 4 (%e1) 2 (%e2) 2 (%e3) 2 for example, 1.76(1.7%) 1.89(1.1%) 1.76 (0.03) 1.89(0.02) 5.64 e 4 5.64 e 4 , or 0.59(3.4%) 0.59 (0.02) %e 4 (1.7%) 2 (1.1%) 2 (3.4%) 2 4.0% answer is 5.64 (4.0%) 4.0% 5.64 0.040 x 5.64 0.23 So we have, 5.6 (0.2) (absolute uncertaint y); 5.6 (4%) relative uncertaint y Its Now Your TURNS unknown material mass 4.635 0.002g volume 1.13 0.05ml (i) Find % relative uncertaini ty in mass and volume (ii) Find the density wi th uncertaint y with correct no. of digits mass Hey, do you remember, density volume Statistics Gaussian Distribution The most probable values occur in the center of the graph, and as you go to either side, the probability falls off Gaussian Distribution Mean (x) : average of the numerical data x i mean, x i n 1 ( x1 xx 2 x3 ... xn ) n Standard deviation (s) : a measure of the width of the distributi on standard deviation, s 2 ( x i x) i n 1 Gaussian Distribution For Gaussian curve representing an “infinity” number of data set, we have (mu) = true mean (sigma) = true standard deviation For an ideal Gaussian distribution, about 2/3 of the measurements (68.3%) lie within one standard deviation on either side of the mean. Student’s t - Confidence Intervals From a limited number of measurements, it is impossible to find the true mean, , or the true standard deviation, . What we can determine are x and s, the sample mean and the sample standard deviation. The confidence intervals is a range of values within which there is a specified probability of find the true mean Student’s t - Confidence Intervals ts Confidence Interval : x n t can be obtained from “Values of Student's t table” see textbook, pp.78 “Q-Test” for Bad Data What to do with outlying data points? Accept? Or Reject? How to determine….. gap Q - test for discarding data : Q range “Q-Test” for Bad Data gap 1.4 Qcalcu lated 0.37 range 3.8 If Q(cal.) Q(tabulate d), the questionab le value should be discarded. In this case, Q (cal.) Q(tabulate d), thus the data point should be retained. Q (90% confidence) Number of observations 0.76 0.64 0.56 0.51 0.47 0.44 0.41 0.39 0.38 0.34 0.30 4 5 6 7 8 9 10 11 12 15 20 “Q-Test” for Bad Data Q (90% confidence) Number of observations 0.76 0.64 0.56 0.51 0.47 0.44 0.41 0.39 0.38 0.34 0.30 4 5 6 7 8 9 10 11 12 15 20 Least-Square Analysis (Linear Regression) Least-Square Analysis (Linear Regression) Finding “the best straight line” through a set of data points Equation of a straight line: y = mx + b m = slope; b = y-intercept vertical deviation di yi - y yi - (mx i b) di 2 (yi - y) 2 (yi - (mx i b)) 2 Least-Square Analysis (Linear Regression) Least - squares slope : m n ( xiyi ) xi yi D 2 ( x i ) yi ( xiyi) xi Least - squares intercept : b Denominato r, D D n ( xi 2 ) ( xi ) 2 D Least-Square Analysis (Linear Regression) standard deviation, sy (deviation of each yi from the centre of the Gaussian curve) sy standard deviation of slope : standard deviation of intercept : s m sy sb sy 2 ( d i ) n2 n D 2 ( x i ) D Calibration Curve Calibration Curve is a graph showing how the experimentally measured property (e.g. absorbance) depends on the known concentrations of the standards A solution containing a known quantity of analyte is called a standard solution Calibration Curve Calibration Curve From the calibratio n curve, we will obatined the seeked - value, x The uncertaint y in x from calibratio n curve is 2 2 sy x n ( xi ) 2 x xi uncertaint y in x 1 m D D D