Properties and Classification 2015 Studyguide Worksheet

Studyguide Worksheet
N/D/CP
__________________________________________
Use the FREE NOTES SHEET, “Describing and Classifying Matter” and your textbook.
Test is Tuesday of next week.
Matter Basics - “Like duh” Questions
1. The universe is made of ________ and _energy________.
2. Matter is anything with what two properties?
3. Chemistry is the study of what two things?
Classification of Matter - Thinking Required
1. Why bother classifying matter? Okay, so maybe this wasn’t in the notes and won’t be on the test. Just curious if you can figure out a logical reason why we would need to know this. There are a few, by the way.
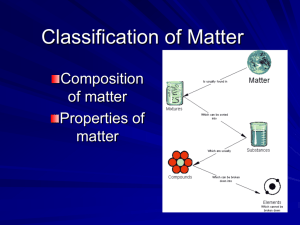
2. All matter is divided into what two big groups?
3. If your ball of gloopidydo is made of two or more substances physically combined (meaning, the different atoms aren’t connected, they just float around each other) , then is your gloopidydo a mixure or a compound?
4. If your ball of gloopidydo is made of two or more substances chemically combined (meaning, the atoms are connected by their electrons), then is your gloopidydo a mixture or a compound?
5. What’s the difference between a molecule and a compound?
6. Are they both pure substances?
7. Is an element a pure substance or mixture?
8. What are the two main types of mixtures? Explain how they differ from each other.
9. Is a solution a homogeneous or heterogeneous mixture?
10.What is the difference between a solute and a solvent?
11. If one liquid is dissolved into another, like mixing ice tea and lemonade together, how do you know which is the solute and which is the solvent? (Tough one!)
12. What make a colloid different from a regular heterogeneous mixture?
13. What makes a suspension different from a regular heterogeneous mixture?
14. A foam is a colloid with ______ particles floating in a ________ or a _________.
15. An aerosol is a colloid with ________ particles floating in a ________.
16. A sol is a colliod with ___________particles floating in _____________.
17. An emulsion is a colloid with _____________ particles floating in ______________.
18. How many angels can dance on the head of a pin?
19. What? That’s not a scince question.
20. Well, just guess then.
21. This is stupid.
22. Aw, c’mon. Really, how many angels can dance on the head of a pin?
23. How can I guess about the number of something with no prior research data?
24. Whatever, it’s just a question.
25. Can we just get back to the studyguide?
26. Two immiscible liquids cannot do what?
27. Those liquids above can do that impossible thing, however, with the help of an...
28. Mayonnaise is an example of an ...
ickky, nasty-tasting, thigh fattening goo or what type of colloid?
29. If something is insoluable, it cannot do what?
30. Explain the difference between an unsaturated, saturated, and supersaturated solution.
Identification
– Labeling
Label each item as an element , a compound/molecule , a homogenous mixture or a heterogeneous mixture .
1. copper
2. salt
3. milk
4. Oreo cookies
5. what you barffed up when ate too many Oreo cookies
6. krypton gas
7. granite
8. a gummi bear
9. a gummi bear that has had it’s head torn off and replaced with another color bear head
10. sugar
11. carbon dioxide
12. water
Physical and Chemical Properties
1. First of all, what’s a property? ( We keep using this word - do you know what it means?
)
2. A physical property describes what about an object?
3.A chemical property describes what about an object?
4. List as many physical properties as you can. Color, etc.
5. List as many chemical properties as you can.
6. Give four physical properties of a gummi bear and two chemical properties of a gummi bear.
7.What kind of property describes just the thing itself?
8. What kind of property describes the thing by how it reacts with other things?
9. Does a physical change change the substance at the molecular level? Does a chemical change do it? No. Yes.
10. A _ chemical ___ change ALWAYS creates a new substance.
11. Be creative. Pick your favorite furry woodland creature. (like a wombat) Describe its physical and chemical properties and give two physical changes and one chemical change you could do to it.
Physical properties -
Chemical properties -
Physical changes – cutting, smashing, melting, boiling, freezing, diluting (none are very nice)
Chemical change
– digesting it, burning, reacting with acids, reacting with bases (also, none very nice
Physical Property Specifics - Phase change and Density
1. How do you change one phase to another?
2. What happens to the temperature of a substance as it goes through phase change?
3. Define viscosity.
4. If your spit has a high viscosity, is it thick or thin?
5. Describe all the phase changes ( ie, solid to a gas = sublimatio n)
6. Define density and words and with its formula.
7. T/F. A tiny block of lead has the same density as a Manhatten-size piece of lead.
8. The mass of a 900 gram ferret that has a volume of 100 centimeters cubed would be...?
9. What are two common units for density?