01) Solubility, Dissociation, and Molarity - chem30-wmci
advertisement

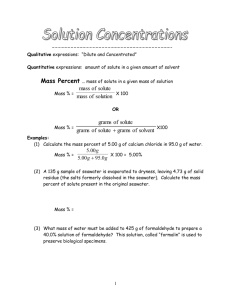
CHEMISTRY 30 – UNIT 2 – SOLUBILITY SOLUBILITY DISSOCIATION & MOLARITY SOLUTIONS – ESSENTIAL INFO • A solution is two or more substances combined to form a homogeneous mixture • Recall: • homogeneous mixture is uniform and the individual parts of the mixture are indistinguishable. • ex) sea water, air, glass • heterogeneous mixtures where you can see the separate components of the mixture. • ex) sand and water SOLUTIONS - COMPONENTS • In any given solution, there are always the following two parts: • i. Solvent: this is the most abundant substance. Typically considered to be the thing that a substance is dissolved in • ii. Solute: this is the least abundant substance(s). Typically, it is considered to be the thing that is dissolved. SOLUTIONS - STATES • Solutions can be solid, liquid, or gas, or a combination of two or more states. • Liquid: • vinegar = acetic acid and water • Gas • air = nitrogen, oxygen, argon, carbon dioxide, etc. • Solid • brass = copper and zinc , steel = iron and carbon • Solid and liquid • salt water, iced tea, etc. • Gas and liquid • Soda; carbonated beverages AQUEOUS SOLUTIONS • An aqueous solution is a solution in which the solvent is water. We will deal with aqueous solutions frequently. • If more solute can dissolve, it is an unsaturated solution • Eventually, if you keep adding a solute, it will not be able to dissolve into the solution anymore. • At this point, we call the solution saturated. AQUEOUS SOLUTIONS • When a solution becomes saturated, it reaches EQUILIBRIUM. • A solution that is at equilibrium means the RATE at which a solute dissolved is equal to the rate at which it drops out of the solution. • For example, a saturated solution of salt and water appears as if undissolved salt sits at the bottom of the solution. • However, at the molecular level, dissolved salt is constantly leaving the water and being replaced by undissolved salt. AQUEOUS SOLUTIONS • When a system is in equilibrium, the rate at which dissolved solute is replaced by undissolved solute is the same. • Equilibrium equations look like: NaCl (s) ⇌ Na+ (aq) + Cl- (aq) SOLUBILITY • Solubility is the quality of a particular substance that can dissolve in a particular solvent (yielding a saturated solution) • Solubility refers to the amount of substance needed to make a saturated solution at a specific temperature. • At a specific temperature, the amount of solid solute capable of dissolving in a given volume of solvent is fixed. • That is, at a given temperature, if too much solute is added it will generally accumulate at the bottom of the solution. DISSOCIATION • When adding water to an ionic compound, a lot of times the water will cause the bonds to break apart in the compound. • This causes individual ions to move freely about. • This process of decomposition into component ions is called dissociation. • ex) Solid magnesium nitrate in water Mg(NO3)2(s) ⇌ Mg2+ (aq) + NO3- (aq) • *Note, there is no need to include water as a reactant DISSOCIATION • Write the dissociation equations for the following compounds • Li2S(s) • Sr(NO3)2(s) FACTORS INFLUENCING SOLUBILITY • In general, an increase in temperature will increase the solubility of solids in liquids, but decrease the solubility of gasses in liquids. • For example, the amount of oxygen in a river is dependant on the temperature of the water. • However, increase in pressure will increase the solubility of gasses. • For example, if pop is pressurized there will be a lot of gas in the pop (it will taste fizzy). Once it is open, the solubility of gas is decreased so gas escapes (it gets flat). CONCENTRATION • THE WORD “CONCENTRATION” REFERS TO HOW MUCH SOLUTE IS DISSOLVED IN THE SOLUTION. YOU GET THE IDEA: DISSOLVING TWO CUPS OF SUGAR IN ONE LITRE OF WATER WILL PRODUCE A MORE CONCENTRATED SOLUTION THAN DISSOLVING ONE CUP OF SUGAR IN ONE LITRE OF WATER WOULD. CONCENTRATION • “CONCENTRATION” CAN BE MEASURED IN A VARIETY OF WAYS. BY FAR THE MOST USEFUL IS MOLARITY. • THE MOLARITY OF A SOLUTION IS CALCULATED BY TAKING THE MOLES OF SOLUTE AND DIVIDING BY THE LITRES OF SOLUTION. MOLARITY • FORMULA IS AS FOLLOWS: 𝑴𝒐𝒍𝒂𝒓𝒊𝒕𝒚 = UNITS: o MOLARITY = MOLAR (M) o MOLES OF SOLUTE = MOLES (mol) o LITERS OF SOLUTION = LITERS (L) 𝑴𝒐𝒍𝒆𝒔 𝒐𝒇 𝑺𝒐𝒍𝒖𝒕𝒆 𝑳𝒊𝒕𝒓𝒆𝒔 𝒐𝒇 𝑺𝒐𝒍𝒗𝒆𝒏𝒕 EXAMPLE #1 • SUPPOSE WE HAD 1.00 MOLE OF SUCROSE (IT'S ABOUT 342.3 GRAMS) AND PROCEEDED TO MIX IT INTO SOME WATER. IT WOULD DISSOLVE AND MAKE SUGAR WATER. WE KEEP ADDING WATER, DISSOLVING AND STIRRING UNTIL ALL THE SOLID WAS GONE. WE THEN MADE SURE THAT WHEN EVERYTHING WAS WELL-MIXED, THERE WAS EXACTLY 1.00 LITER OF SOLUTION. • WHAT WOULD BE THE MOLARITY OF THIS SOLUTION? EXAMPLE #1 • MOLARITY = 1.00 MOL 1.00 L • THE ANSWER IS 1.00 MOL/L. NOTICE THAT BOTH THE UNITS OF MOL AND L REMAIN. NEITHER CANCELS. • A REPLACEMENT FOR MOL/L IS OFTEN USED. IT IS A CAPITAL M. SO IF YOU WRITE 1.00 M FOR THE ANSWER, THEN THAT IS CORRECT. EXAMPLE # 2 • SUPPOSE YOU HAD 2.00 MOLES OF SOLUTE DISSOLVED INTO 1.00 L OF SOLUTION. WHAT'S THE MOLARITY? • MOLARITY = 2.00 MOL 1.00L • THE ANSWER IS 2.00 M. • NOTICE THAT NO MENTION OF A SPECIFIC SUBSTANCE IS MENTIONED AT ALL. THE MOLARITY WOULD BE THE SAME. IT DOESN'T MATTER IF IT IS SUCROSE, SODIUM CHLORIDE OR ANY OTHER SUBSTANCE. ONE MOLE OF ANYTHING CONTAINS 6.02 X 1023 UNITS. EXAMPLE # 3 • WHAT IS THE MOLARITY WHEN 0.750 MOL IS DISSOLVED IN 2.50 L OF SOLUTION? • MOLARITY = 0.750 MOL 2.50 L • THE ANSWER IS 0.300 M. EXAMPLE # 4 • WE CAN CALCULATE THE MOLARITY OF A SOLUTION WHEN GIVEN THE AMOUNT OF SOLUTE IN GRAMS. WE CONVERT FROM GRAMS TO MOLES, AND THEN PROCEED AS IN THE PREVIOUS EXAMPLES. • WHAT IS THE MOLARITY OF A 2.00L SOLUTION THAT HAS 102.44G NaCl DISSOLVED IN IT? • STEP ONE: CONVERT GRAMS TO MOLES. • STEP TWO: DIVIDE MOLES BY LITERS TO GET MOLARITY. GIVEN ANY TWO OF THE VALUES FROM THE MOLARITY CALCULATION, WE CAN EASILY CALCULATE THE THIRD • EXAMPLE #5 – HOW MANY GRAMS OF NaOH ARE CONTAINED IN 1.45 L OF A 2.25 M SOLUTION? • STEP ONE: DETERMINE HOW MANY MOLES OF NaOH ARE PRESENT IN THE SOLUTION. • STEP TWO: CONVERT FROM MOLES TO GRAMS USING THE FORMULA WEIGHT OF NaOH. EXAMPLE # 6 • WHAT VOLUME (IN mL) OF A 0.20 M SOLUTION OF NaOH CONTAINS 1.00 G OF NaOH? • STEP ONE: DETERMINE HOW MANY MOLES ARE IN 1.00G OF NaOH. • STEP TWO: USE YOUR MOLE VALUE ALONG WITH YOUR MOLARITY TO SOLVE FOR VOLUME HERE’S A (SEEMINGLY) MORE COMPLICATED PROBLEM: • EXAMPLE #7 – A 0.050 M SOLUTION OF GLYCERINE, C3H8O3, AND A 0.050 M SOLUTION OF LYCINE, C5H11NO2, ARE PREPARED. WHICH SOLUTION CONTAINS THE MOST DISSOLVED MOLECULES PER LITRE? A (SEEMINGLY) COMPLICATED PROBLEM) THIS LOOKS HARD, BUT IT’S VERY EASY. REMEMBER THAT M MEANS MOLES/LITRE. SINCE WE’RE WORKING WITH ONE LITRE VOLUMES, EACH SOLUTION WILL THEREFORE HAVE 0.050 MOLES OF SOLUTE. EACH SOLUTION WILL ALSO THEREFORE HAVE THE SAME NUMBER OF MOLECULES: MULTIPLY 0.050 MOLES BY AVOGADRO’S NUMBER. STOICHIOMETRY WITH SOLUTIONS • EXAMPLE #8 – HOW MANY GRAMS OF COPPER WILL REACT TO COMPLETELY REPLACE SILVER FROM 208 ML OF 0.100 M SOLUTION OF SILVER NITRATE, AgNO3? YOUR PRODUCT WILL BE COPPER (II) NITRATE. • USE THE SAME STOICHIOMETRY STEPS AS ALWAYS: • 1. GET A BALANCED CHEMICAL EQUATION • 2. TAKE YOUR INFO AND GET TO MOLES • 3. USE THE MOLE RATIOS TO REBALANCE THE EQUATION • 4. GO WHERE THE QUESTION ASKS