The Structure of Atoms
advertisement

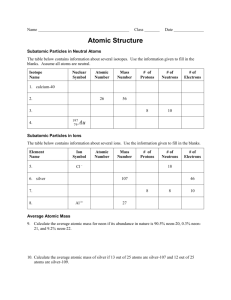
Monte Meyerink Describe the evidence for the existence of electrons, protons, neutrons, and describe the properties of these subatomic particles. Discuss atoms of different elements in terms of their numbers of electrons, protons, and neutrons, and define the terms atomic number and mass number. Experiments by scientists in the mid 19th century led to a change in John Dalton’s atomic theory, which stated that atoms were indivisible and indestructible. They discovered that atoms could be broken down into pieces called subatomic particles. The three most important subatomic particles are the electron, proton, and neutron. Electrons were discovered by J.J Thomson’s use of cathode (electricity) rays. ◦ Rays had a negative charge since they originated from the negatively charged cathode ◦ Rays consisted of tiny particles that were hitting the paddles of the wheel. ◦ A cathode ray consisted of particles that have a very small mass and charge of -1. We call these particles electrons (e-). Electrons exist on the edge of atoms in different shell levels. Scientists began searching for the positively charged area of an atom. Ernest Rutherford used the gold foil experiment to determine that there was a space in the atom that was very dense, concentrated, and positively charged. This region of the atom is called the nucleus. ◦ Contains nearly all of an atom’s mass, but a small fraction of an atom’s volume. ◦ Located at the center of the atom. A proton (p+ or p) is a positively charged particle (charge of +1). The charge of a proton was calculated to be equal in magnitude but opposite in sign to the charge of an electron. The proton’s mass is 2000 times the mass of an electron. Since protons and electrons together did not account for the entire mass of the atom, scientist searched for another subatomic particle. This particle had to be neutral to keep the atom stable. James Chadwick projected alpha particles at elements to discover neutrons (n). Neutrons have a charge of 0 and almost have the exact same mass as protons. Where are the protons, neutrons, and electrons located in an atom? What are the charges of each subatomic particle? What are the relative masses of each particle in regards to the total mass of the atom? Atomic Number: the number of protons that an atom has. ◦ Hydrogen has an atomic number of one since it only has one proton. Carbon Mercury Plutonium Mass Number: the total number of particles in the nucleus (protons + neutrons). ◦ Helium has a mass number of 2, one proton and one neutron. Lead Gold Bromine Describe the evidence for the existence of electrons, protons, neutrons, and describe the properties of these subatomic particles. Discuss atoms of different elements in terms of their numbers of electrons, protons, and neutrons, and define the terms atomic number and mass number.