Acids and Bases
advertisement

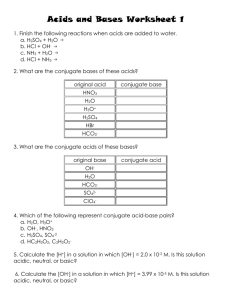
Acids and Bases Roselyn Dooley, Tyler Schmidt, Kyle Doubleday and Deondré Robinson Properties of Acids -Sour taste -React with active metals -Turns litmus paper Red -React with bases to produce salt and water -Conduct electricity -1- 6.9 on pH scale Properties of Bases -Bitter taste -Slippery feel -Turns litmus paper blue -Reacts with acids to produce salt and water -Conduct electricity -7.1 - 14 on pH scale Binary and Tertiary acids Binary acid- an acid that contains only two different elements: hydrogen and one of the more electronegative elements. Tertiary acid- an acid that contains hydrogen oxygen and one more element. Theories of Acids and Bases -Arrhenius Acid- A substance that dissociates to produce hydrogen ions in water Base- A substance that dissociates to produce hydroxide ions in water -Bronsted-Lowry Acid- Any substance that can donate H+ ions. (A proton donor) Base- Any substance that can accept H+ ions. (A proton acceptor) Theories of Acids and Bases Cont. -Lewis Acid- Any substance that can accept a pair of nonbonding electrons. (electron pair acceptor) Base- Any substance that can donate a pair of nonbonding electrons. (electron pair donor) Naming Acids • Rule #1 o • If the negative ion in the acid ends in "ide" you name the acid "Hyrdo (stem) ic acid Ex: HCl (Chloride) would yield Hydrochloric acid Rule # 2 o If the negative ion in the acid ends in "ite", you name the acid "(stem)ous acid" Ex: HNO2 (Nitrite) would yield Nitrous Acid *Use only if there is an oxygen in the chemical formula Naming Acids Cont. • Rule # 3 o If the negative ion ends in "ate", you name the acid "(stem)ic" acid. Ex: HIO4 (Periodate) would yield Periodic acid Note: The stem of Sulfur is Sulfur • Also the Stem of Phosphor is phosphor Name that Acid! 1. HSCN 2. HClO2 3. HClO3 4. HBr 5. H2SO3 6. H3P Answers 1. Thiocyanic acid 2. Chlorous acid 3. Chloric acid 4. Hydrobromic acid 5. Sulfurous acid 6. Hydrophosphoric acis pH Scale Six Strong Acids • • • • • • HCl HBr HI HNO3 H2SO4 HClO4 o Everything else is considered a weak acid Writing Acid-base reactions in aqueous solutions General Formulas Strong Acids Weak Acids HA H+1 + A-1 HA +H2O H3O+1 +A-1 HCl H+1 + Cl-1 HF + H2O H3O+1 + F-1 Now you try Write the acid base reactions in aqueous solutions 1. HBr 2. H2SO4 3. HCN 4. HC2H3O2 Answers 1. HBr H+1 + Br-1 2. H2SO4 H+1 + SO4-2 3. HCN + H2O H3O+1 + CN-1 4. HC2H3O2 + H2O H3O+1 + C2H3O2-1 Neutralization Reactions between Acids and Bases Neutralization- The reaction of hydronium ions and hydroxide ions to form water molecules Example equationHCl(aq) + NaOH(aq) NaCl(aq)+ H2O(l) Note: This is basically just a double displacement reaction Now you try 1. HClO4(aq)+ NaOH(aq) 2. HBr(aq) + Ba(OH)2 (aq) ***You might need your pink sheet Answers 1. HClO4(aq)+ NaOH(aq) 2. 2HBr (aq)+ Ba(OH)2 (aq) 2H2O(l) NaClO4(aq)+ H2O(l) BaBr2 (aq) + Calculate Hydronium and Hydroxide [H+]=10-pH ex. [H+]=10-4 [H+]=1 x 10-4 M [OH-]=10-pOH ex. [OH-]=10-7.5 [OH-]=3.16 x 10-8 M 1. pH= 2.2 1. pOH=7.8 2. pH=3.6 2. pOH=9.3 3. pH=8.8 3. pOH=5.6 4. pOH=9 4. pH=3 Hydronium and Hydroxide Answers 1. [H+]= .0063 M 1. [OH-]= 1.58 x 10-8 M 2. [H+]= 2.51 x 10-4 M 2. [OH-]= 5.01 x 10-10 M 3. [H+]= 1.58 x 10-9 M 3. [OH-]= 2.51 x 10-6 M 4. [H+]= 1 x 10-9 M 4. [OH-]= .001 M Calculate pH and pOH pH=-log[H+] ex. pH=-log[2.33 x 10-9 M] pOH=-log[OH-] ex. pOH=-log[7.65 x 10-3 M] pH=8.63 1. [H+]=7.24 x 10-5 M 2. [H+]=6.32 x 10-2 M 3. [OH-]=2.26 x 10-8 M 4. [H+]=4.54 x 10-3 M pOH=2.12 1. [OH-]=5.58 x 10-4 M 2. [OH-]=3.67 x 10-8 M 3. [OH-]=2.77 x 10-2 M 4. [H+]=4.49 x 10-7 M pH and pOH Answers 1. pH= 4.14 1. pOH= 3.25 2. pH= 1.2 2. pOH= 7.43 3. pH= 6.35 3. pOH= 1.56 4. pH= 2.34 4. pOH= 7.65 Titration The controlled addition and measurement of the amount of a solution of known concentration required to react completely with a measured amount of a solution of unknown concentration. Once the two solutions are chemically equivalent, the solution has reached the equivalence point. Titration Essentially, you add an acid of known molarity (concentration) to a base of unknown molarity in measured amounts to find the unknown, or vice versa. Once at the equivalence point, the unknown concentration can be calculated using known concentration and volumes. Walkthrough Problem 500 mL of .5 M HF titrates with 635 mL NaOH. 1) Balance the equation: HF + NaOH NaF + H2O 2) Choose method: M1V1 = M2V2 or conversions 3) (.5M) (.500 L) = (x M) (.635 L) 4) .5 M (.500 L) .635 L 5) .4 M NaOH Titration Calculations Ex 1) 25 mL of .3M HCl reaches an equivalence point with 75 mL of NaOH. What is the molarity of the NaOH? Titration Calculations 1) Balance the equation: HCl + NaOH NaCl + H2O 2) Because the mole ratios are equal (1:1), we can use the formula M1V1 = M2V2 3) (.3M) (.025 L) = (x M) (.075 L) 4) .3 M (.025 L) =xM .075 L 5) .1 M NaOH Titration Calculations Ex 2) 550 mL of H2SO4 of unknown concentration reaches an equivalence point with 775 mL of 2.0 M NaOH. What is the concentration of the H2SO4. Titration Calculations 1) Balance the equation: H2SO4 + 2NaOH Na2SO4 + 2H2O 2) Because the mole ratios are not equal (1:2), we must use conversion factors. 3) 2.0 M NaOH = (x) mols NaOH .775 L NaOH 4) 1.55 mols NaOH x 1 mol H2SO4 = .755 mols H2SO4 2 mols NaOH 5) .755 mols H2SO4 = 1.4 M H2SO4 .550 L H2SO4 Questions for us Sources http://www.google.com/imgres?imgurl=http://www.dartmouth.edu/~chemlab/techniques/graphics/titration/titration10.gif&imgrefurl= http://www.dartmouth.edu/~chemlab/techniques/titration.html&h=263&w=190&sz=32&tbnid=ZR_ZcE0XRTe1TM:&tbnh=90&t bnw=65&zoom=1&usg=__Lx_UdDHNNq5SJQMUUATJ_Hg7nCc=&docid=8iiaXS9UV6QRhM&sa=X&ei=gF6tUfDeDtCJ0QH Fg4DgCA&sqi=2&ved=0CF4Q9QEwBg&dur=155 http://www.elmhurst.edu/~chm/vchembook/184ph.html http://copernicusconsulting.net/designers-are-not-researchers-the-difference-between-design-and-social-research/ http://www.thechemicalblog.co.uk/what-is-a-titration/ http://dl.clackamas.edu/ch104-04/double.htm http://www.pc.maricopa.edu/data/GlobalFiles/file/chemistry/lee/Double%20displacement%20reaction.pdf https://www.google.com/url?sa=i&rct=j&q=&esrc=s&source=images&cd=&cad=rja&docid=bZiz86EUg2v9FM&tbnid=h7bsJowJO9 PdSM:&ved=0CAUQjRw&url=http%3A%2F%2Fthepickledhedgehog.com%2F2012%2F02%2F21%2Fsciencelife%2F&ei=Rl-tUYjpM8aw0AGWpoCQAw&bvm=bv.47244034,d.dmQ&psig=AFQjCNH5PXKRxbEbTHb1ihQtytljDNj1Q&ust=1370403008833830