Temperature and Thermal Energy
advertisement

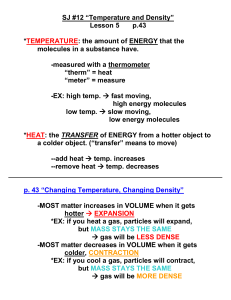
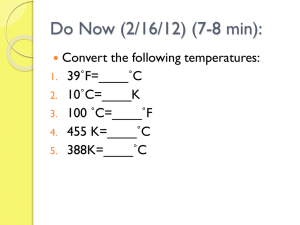
Chapter 12.1 1. Everything is made of particles 2. These particles move 3. Hot things move faster than cold things Temperature – a measure of average kinetic energy of an object Thermometer - device that measures temperature (and, as a result, average KE) Always from hot to cold objects Heat flow stops only when all objects are in equilibrium (at the same temp) If A is 75 degrees and B is 25 degrees, at which temperature are they both in equilibrium? To solve, first add up the different temperatures Next, divide the total temp by 2 (because we have 2 objects) This is the equilibrium temperature Answer = 50 degrees The temp at which molecular motion stops Is this cold or hot?? Know how to do these… 50°C = _______ K 325 K = ________°C Energy transfer when objects are in direct contact. There must be matter in order for energy to transfer by conduction. Energy transfer by air or water currents. Objects are not in direct contact Look for currents Know that hot air and water rises!! Matter is required for heat transfer by convection Energy transfer thru empty space No matter is required!! Its how the sun heats the Earth Sunbathers are often used as examples of heating by radiation Q = CmΔT Q = heat gained or lost (J) m = mass in kg C = specific heat (see p.279) ΔT = temp change (K or °C) Tf = maCaTai maCa + mbCbTbi + mbCb How much heat is needed to raise the temperature 1.5 kg of water from 22°C to 35°C? m = 1.5 kg ΔT = 15°C C = ?? What is changing temperature? Use the specific heat for whatever is changing temp (see page 279) Q = 4180 x 1.5 x 15 Q = 94,050 J A 5.0 kg sample of water at 25°C is mixed with 1.5 kg of water at 75°C. What is the final temperature of the mixture? Use the Tf formula… Tf = 5 x 4180 x 25 5 x 4180 + + 1.5 x 4180 x 75 1.5 x 4180 Tf = 459800 20900 + + 470250 6270 Tf = 34°C Page 296 #’s 23 – 24 and 27-28