Hyponatremia
advertisement
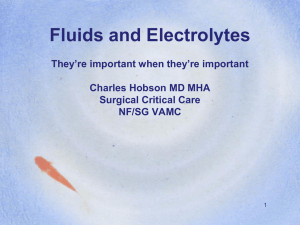
Osmolality vs Effective Osmolality Osmolality: total number of particles in an aqueous solution (mosmol/kg H2O) Normal Posm = 275-290 mosmol/kg Effective osmolality (tonicity): those particles that can exert osmotic force across membranes, via movement of water into or out of cells Examples: Na+, glucose, mannitol Normal effective Posm = 270-285 mosmol/kg Plasma Osmolality Na+, glucose and BUN are major determinants of plasma osmolality Posm = 2 x plasma [Na+] + [Glucose]/18 + [BUN]/2.8 More important clinically to consider effective osmolality than “total’’ osmolality Effective osmoles (Na+ , glucose) exert water shifts unlike urea (as well as ethanol) Plasma Osmolality Is hyponatremia always associated with a low plasma osmolality? NO Plasma Osmolality Example Serum Na+ = 125 mEq/L BUN = 140 mg/dL Blood glucose = 90 mg/dL Calculated and measured osmolality = 305 mOsm/kg Posm = 2 x 125 + 90/18 + 140/2.8 In this case, hyponatremia is associated with an elevated plasma osmolality Effective osmolality = 255 mOsm/kg (calculation excludes BUN) thus this patient may have symptoms of hypotonicity despite an elevated plasma osmolality Plasma Osmolality Is plasma hypoosmolality always associated with hyponatremia? YES Posm ~ 2 x plasma [Na+] Plasma Osmolality Is hyponatremia always associated with hypotonicity? NO Plasma Osmolality Example: Serum Na+ = 133 mEq/L BUN = 11 mg/dL Blood glucose = 500 mg/dL Effective osmolality (tonicity) = 294 mOsm/kg (2 x 133 + 500/18) Hyponatremia is not always associated with hypotonicity and thus direct therapeutic intervention may not be required (in this example, treat underlying hyperglycemia) Plasma Osmolality Do ineffective osmoles (urea, ethanol, ethylene glycol, methanol cause hyponatremia)? NO. Remember these osmoles readily move between fluid compartments without causing water shifts Plasma Osmolality Do effective osmoles (glucose, mannitol) cause hyponatremia? Yes. These osmoles shift water out of the cells Clinical Examples of Hyponatremia Plasma Na+ = 120 mEq/L Blood glucose = 90 mg/dL BUN = 14 mg/dL Calc Posm = 250 mosmol/kg Hypotonic hyponatremia Meas Posm = 250 mosmol/kg Osmolar gap = 0 mosmol/kg Tonicity = 245 mosmol/kg risk of cerebral edema Clinical Examples of Hyponatremia Plasma Na+ = 120 mEq/L Blood glucose = 90 mg/dL BUN = 14 mg/dL Calc Posm = 250 mosmol/kg Pseudohyponatremia ( lipids, protein) Note: absence of osmolar gap rule out this diagnosis Meas Posm = 290 mosmol/kg Osmolar gap = 40 mosmol/kg Tonicity = 285 mosmol/kg No risk of cerebral edema Clinical Examples of Hyponatremia Plasma Na+ = 120 mEq/L Blood glucose = 1350 mg/dL BUN = 14 mg/dL Calc Posm = 320 mosmol/kg Hyponatremia caused by hyperglycemia Meas Posm = 320 mosmol/kg Osmolar gap = 0 mosmol/kg Tonicity = 315 mosmol/kg No risk of cerebral edema Clinical Examples of Hyponatremia Plasma Na+ = 120 mEq/L Blood glucose = 90 mg/dL Hyponatremia caused by mannitol BUN = 14 mg/dL [Mannitol] = 75 mmol/L Calc Posm = 250 mosmol/kg Meas Posm = 325 mosmol/kg Osmolar gap = 75 mosmol/kg Tonicity = 320 mosmol/kg Osmolar gap (≠ hyperglycemia) No risk of cerebral edema Clinical Examples of Hyponatremia Plasma Na+ = 120 mEq/L Blood glucose = 90 mg/dL BUN = 14 mg/dL Calc Posm = 250 mosmol/kg Hyponatremia due to ethanol [EtOH] = 50 mmol/L Meas Posm = 300 mosmol/kg Osmolar gap = 50 mosmol/kg Tonicity = 245 mosmol/kg risk of cerebral edema Clinical Examples of Hyponatremia Plasma Na+ = 120 mEq/L Blood glucose = 90 mg/dL BUN = 126 mg/dL Calc Posm = 290 mosmol/kg Meas Posm = 290 mosmol/kg Osmolar gap = 0 mosmol/kg Tonicity = 245 mosmol/kg Hyponatremia caused by renal failure risk of cerebral edema Note: a normal measured plasma osmolality does not preclude an increased risk of cerebral edema Causes of Hyponatremia Current Medical Diagnosis & Treatment, 2009 Hypotonic Hyponatremia Hypovolemic ↓ [Na+] = ↓↓TBNa/↓TBW Euvolemic ↓ [Na+] = ↔ TBNa/↑TBW Hypervolemic ↓ [Na+] = ↑TBNa/↑↑TBW Laboratory Approach to Hyponatremia Start with plasma osmolality to exclude pseudohyponatremia (normal Posm) and hypertonic hyponatremia (elevated Posm) When hypotonicity is confirmed, then assess clinically patients’ volume status Urine Osmolality Determine whether H2O excretion is normal or impaired Uosm < 100 mosmol/kg indicates that ADH is appropriately suppressed Primary polydipsia Reset osmostat (when Posm is below normal) Low solute intake Uosm > 100 mosmol/kg occurs in majority of hyponatremic patients and indicates impaired H2O excretion Urine Sodium Concentration Una < 20 mEq/L Hypovolemia due to extra-renal losses Edematous states in CHF, cirrhosis, nephrotic syndrome Dilutional effect in primary polydipsia due to very high urine output Una > 20 mEq/L Hypovolemia due to renal losses Renal failure SIADH Reset osmostat Other Labs Plasma uric acid concentration Hypouricemia (< 4mg/dL) in SIADH Mild hypervolemia decreases proximal Na+ reabsorption, leading to increased urinary uric acid excretion Blood urea nitrogen BUN may be < 5mg/dL in SIADH Mild hypervolemia leads to urinary urea wasting Case Illustration 1 62 year old woman was admitted to the hospital for abnormal liver-function tests. She had a history of acute myelogenous leukemia and had undergone transplantation of T-cell depleted allogeneic bone marrow 2 years earlier. Medications include tacrolimus, prednisone, MMF, ursodiol, atovaquone, acyclovir and clarithromycin. Exam: afebrile, BP 130/75, HR 80. Appeared euvolemic. Labs revealed serum sodium level 124 mmol/L. What labs do you want to order? NEJM 2003; 349:1465-9 Case Illustration 1 Serum osmolality 294 mOsm/kg Urine osmolality 434 mOsm/kg Urine sodium 62 mmol/L BUN 43 mg/dL Serum creatinine 1.4 mg/dL Serum glucose 85 mg/dL Calculated plasma osmolality 268 mOsm/kg Case Illustration 1 Total serum protein 5.1 gm/dL Lipemia was not observed Lipid profile (2 years prior) Total cholesterol 181 mg/dL Triglyceride 136 mg/dL What would you do next? Case Illustration 1 Current lipid profile Total cholesterol 1836 mg/dL High-density lipoprotein 68 mg/dL Very low-density lipoprotein 42 mg/dL Triglyceride 208 mg/dL Calculated low-density lipoprotein 1726 mg/dL Serum [Na+] was 145 mmol/L when measured on a blood-gas machine What’s the cause for the patient’s hyponatremia? Severe hypercholesterolemia causing pseudohyponatremia Lipoprotein X Reflux of unesterified cholesterol and phospholipids into the circulation from cholestatic biliary ducts These cholesterol particles are insoluble in plasma water and thus increase the solid fraction of plasma Occurs in patients with severe cholestasis (chronic graft- versus-host disease, primary biliary cirrhosis) Serum is NOT lipemic (≠ severe hypertriglyceridemia) Pseudohyponatremia Each liter of plasma contains ~ 930 ml water ~ 70 ml proteins and lipids High lipids or proteins reduce plasma water; thus plasma [Na+], measured per liter of plasma, is artifactually low Plasma osmolality is unaffected Osmometer measures only the Na+ Measurement by an osmometer activity in the plasma water What is the normal physiologic sodium concentration? ~ 151 mEq/L plasma water Pseudohyponatremia Serum [Na+] = 140 mEq/L Serum [Na+] = 130 mEq/L Solids 7% 1 liter plasma 1 liter plasma Solids 14% HYPERLIPIDEMIA Water 93% HYPERPROTEINEMIA Na+ 140 mEq in 930 ml Water 86% OSMOLALITY Measures solute per unit plasma water 140 mEq/930 ml = 151 mEq/liter = 130 mEq/860 ml Na+ 130 mEq in 860 ml Measurements of Serum + [Na ] Flame emission spectrophotometer (FES) Measures serum [Na+] Ion-selective electrode (ISE) Measures [Na+] in plasma water Two methods: Direct potentiometry (using undiluted serum sample) Blood gas machine Indirect potentiometry (using diluted serum sample) Pseudohyponatremia can occur with FES or indirect potentiometry, but not direct potentiometry Flame Emission Spectrophotometer Ultrafine spray of diluted serum sample is blown across a flame Measures the intensity of the light emitted at the wavelength characteristic of sodium Intensity is directly proportional to the # sodium atoms in the sample Sample is compared to a standard aqueous solution of known [Na+] Ion-Selective Electrode Measures electrical potential across a sodium-selective membrane immersed in the serum sample Electrical potential is a function of the Na+ activity in the sample, which correlate with sodium concentration in serum water (in undiluted serum) Case Illustration 2 43-year old woman with persistent renal failure two months after a liver transplant, developed acute hyponatremia during treatment of thrombocytopenia with intravenous immune globulin (in 10% maltose). Before therapy, her serum [Na+] was stable at 131 mmol/L. One gm/kg of IVIG was administered over 12 hours on 2 successive days. After the second infusion, her serum [Na+] was 118 mmol/L. After 4 hours of hemodialysis, the serum [Na+] was 133 mmol/L. Hyponatremia recurred during each of the four successive infusions of IVIG. Direct potentiometry was used for the sodium assay. What’s the cause for this patient’s hyponatremia? Case Illustration 2 Annals of Internal Medicine 1993;118:526-8 Hypertonic Hyponatremia Effective osmoles result in water movement out of cells, decreasing plasma [Na+] by dilution Causes Hyperglycemia-most common Mannitol Sorbitol Glycerol Radiocontrast agents IVIG Causing Hyponatremia Hypertonic hyponatremia due to maltose intoxication Maltose given intravenously is normally metabolized by maltase in the renal proximal tubule and excreted in the urine Metabolic products of maltose metabolism can accumulate in the setting of renal failure, raising the plasma osmolality and causing dilutional hyponatremia Pseudohyponatremia IVIG increases the protein-containing nonaqueous phase of plasma* *NEJM 1998; 339:632 Case Illustration 3 Late on the afternoon of 1 June 1981, a 46 year old woman was admitted in a coma to a hospital in Durban, South Africa. Before dawn that day, she had begun a 90 km marathon race. But 20 km from the finish line, she failed to recognize her husband who had come to assist her. He convinced her to stop running and drove her to the hospital. There she received two liters of IV fluid but then suffered a grand mal seizure and lapsed into coma. Serum [Na+] was 115 mmol/L. CXR showed evidence of pulmonary edema. First case report of exercise-associated hyponatremia complicated by encephalopathy and non-cardiogenic (neurogenic) pulmonary edema Br J Sports Med 2006;40:567-72 Case Illustration 3 What would be your immediate treatment for this patient’s hyponatrema? I would give her 100 ml of 3% saline over 10 minutes Acute Symptomatic Hyponatremia (<48 hours): Treatment Immediate goal: ↑ [Na+] by 1-2 mEq/L/hr using 3% NS ± Lasix 3% NS infusion at 1-2 ml/kg/hour or 100 ml of 3% NS over 10 minutes, raising serum [Na+] 2-3 mEq/L in a short period of time If neurologic symptoms persist or worsen, can repeat 100 ml bolus 1 or 2 more times at 10-minute intervals Aim for cessation of neurologic symptoms, then reduce correction rate Goal increase in serum [Na+] First 24 hours: < 8-10 mEq/L First 48 hours: < 18 mEq/L Why the urgency to treat? minutes hours days NEJM 2000; 342:1581-9 Symptoms of Hyponatremia Signs and symptoms < 125-130 mEq/L: nausea, vomiting (earliest findings) < 115-120 mEq/L: headache, lethargy, obtundation < 110-115 mEq/L: seizures, coma, respiratory arrest Severity of neurologic dysfunction (cerebral edema) is related to the rapidity of decline and level of plasma Na+ concentration Cerebral edema occurs primarily with rapid (over 1-3 days) reduction in plasma [Na+] Exercise-Associated Hyponatremia Occurrence of hyponatremia (< 135 mEq/L) during or up to 24 hours after prolonged physical activity Risk Factors for EAH Excessive drinking (>1.5 L/hr) during event- major risk Exercise duration > 4 hrs or slow exercise pace Low body weight (overhydration in proportion to size) Female gender (may be explained by lower body weight) Pre-exercise overhydration Abundant availability of drinking fluids at event NSAIDS (not all studies) Extreme hot or cold environment EAH: Overhydration Increased fluid intake associated with substantial weight gain during the activity increases risk of hyponatremia Athletes who gained > 4% body weight during exercise had a 45% probability of developing hyponatremia However, excessive fluid consumption is not the sole explanation for development of EAH Hyponatremia did not develop in 70% of the athletes who overconsumed fluids and had an increase in body weight Proc Natl Acad Sci USA 2005; 102: 18550-5 Figure 1. Pathophysiologic factors in the development of exercise-associated hyponatremia (EAH) Rosner, M. H. et al. Clin J Am Soc Nephrol 2007;2:151-161 Therapy of EAH Mild, asymptomatic hyponatremia (130-135 mEq/L) Fluid restriction and observation until spontaneous diuresis occurs Avoid IV 0.9% normal saline due to risk of worsening hyponatremia Severe, symptomatic hyponatremia Hypertonic saline (3% NS) No cases of osmotic demyelination have been reported with treatment of EAH Indicated in patients manifesting encephalopathy and noncardiogenic pulmonary edema Vaptans-no data to indicate efficacy EAH: Neurogenic Pulmonary Edema Exercise-Associated Hyponatremia: Why Are Athletes Still Dying? Moritz, Michael; Ayus, Juan Mechanism of non-cardiogenic pulmonary edema in exercise-associated hyponatremia. Clinical Journal of Sport Medicine. 18(5):379-381, September 2008. DOI: 10.1097/JSM.0b013e31818809ce EAH: Neurogenic Pulmonary Edema A depiction of the Ayus-Arieff syndrome. Hyponatremia produces cytotoxic cerebral edema which in turn leads to a neurogenic pulmonary edema. Pulmonary edema leads to hypoxia, which impairs brain cell volume regulation, resulting in a vicious cycle of worsening cerebral edema and pulmonary edema. This syndrome can be reversed by the prompt administration of 3% NaCl. Exercise-Associated Hyponatremia: Why Are Athletes Still Dying? Moritz, Michael; Ayus, Juan Clinical Journal of Sport Medicine. 18(5):379-381, September 2008. DOI: 10.1097/JSM.0b013e31818809ce Prevention of EAH Avoid over consumption of fluids before, during and after exercise Drink only according to thirst and no more than 400-800 ml per hour Monitor body weight to avoid weight gain No evidence that sports drinks can prevent EAH Most drinks have sodium content 10-20 mEq/L (hypotonic) No evidence that sodium supplementation can prevent EAH Case Illustration 4 70 year old woman was admitted for coronary angiography after developing chest pain. Mild HTN had been detected 8 months previously, followed by treatment with HCTZ. On admission, serum sodium was normal at 140 mEq/L. Weight 70 kg. After the catheterization, pt was encouraged to increase her fluid intake, and over the next 24 hours she drank 5 L of water. On HOD #3, serum sodium was 127 mEq/L. On the following day, she underwent an angioplasty and was once again advised to increase her fluid intake. The next morning the patient complained of fatigue, nausea, headache and dizziness; BP 95/60. Serum sodium was 118 mEq/L. SMJ 1986; 79: 1456-7 Case Illustration 4 What labs would you order? Serum osmolality 253 mOsm/kg Urine sodium 57 mEq/L Urine osmolality 525 mOsm/kg How would you treat this patient? Chronic Symptomatic Hyponatremia (> 48 hrs or of unknown duration) Increase serum [Na+] by 0.5 to 1.0 mEq/L per hour Goal increase in serum [Na+] First 24 hours: < 8-10 mEq/L First 48 hours: < 18 mEq/L What fluid would you use, to be infused at what rate? Hypotonic Hypovolemic Hyponatremia Calculate sodium deficit Na+ deficit = TBW x Na+ deficit per liter Male: TBW = 0.6 (wt in kg) Female: TBW = 0.5 (wt in kg) Amount of sodium required to raise plasma [Na+] from 118 mEq/L to 124 mEq/L: Na+ deficit = 0.5 (70kg) x (124 mEq/L – 118 mEq/L) = 210 mEq Volume of 0.9% NS required for correction (210 mEq) x (1 liter/154 mEq Na+) = 1.36 liter Hypotonic Hypovolemic Hyponatremia Rate of correction Na+ deficit per liter/appropriate rate of correction (124 mEq/L – 118 mEq/L)/0.5 mEq per L per hr = 12 hours 1.36 L normal saline/12 hours = 113 ml per hour Caveats This calculation does not account for continued volume losses during the treatment period As hypovolemia improves with 0.9% NS, ADH release will be appropriately suppressed, resulting in rapid excretion of the excess free water with risk of overcorrection and osmotic demyelination Thiazide-Induced Hyponatremia: Pathogenesis Thiazides, by acting in the cortex in the distal tubule, do not interfere with medullary function and thus with ADHinduced water retention Cause urinary loss of solutes in excess of water Thiazide-Induced Hyponatremia: Pathogenesis Underlying tendency to increase water intake (polydipsia) Impaired water excretion-2 different mechanisms: Volume depletion stimulates release of ADH Increased water retention independent of ADH Via reduced ability to excrete a water load (unclear mechanism), leading to slight volume expansion These patients can develop thiazide-induced hyponatremia despite NOT being volume depleted Can have normal BUN, creatinine and hypouricemia Initial weight gain Thiazide-Induced Hyponatremia Most likely to occur in older women Hyponatremia develops within the first 1-2 weeks of therapy Thiazides rarely cause severe hyponatremia associated with encephalopathy/seizures Case Illustration 5 62 year old woman noted an unpleasant, sweet taste in her mouth. She otherwise felt well and was taking no medications. Because dysgeusia is a rare manifestation of hyponatremia, her serum sodium level was tested and was 122 mEq/L. The serum osmolality was 250 mOsm/kg, the urinary osmolality 635 mOsm/kg and urinary sodium 85 mEq/L. Her thyroid function and adrenal function were normal. A chest CT showed a mass in the lower lobe of the left lung, which proved to be a small-cell carcinoma. What’s the cause of this patient’s hyponatremia and your approach to therapy? Chronic Asymptomatic Hyponatremia: Treatment Correct hyponatremia very gradually Patients are at low risk of serious neurologic sequelae but at risk of osmotic demyelination with rapid correction Fluid restriction is the mainstay of therapy Adequate intake of dietary protein and salt Urine output = solute excretion per day (mosmol)/urine osmolality (mosmol/kg) Demeclocycline 300-600mg po BID Reduces urine osmolality Can be nephrotoxic Urea 30 g po per day Poorly tolerated Syndrome of Inappropriate Antidiuresis (SIAD) Since not all patients with the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) have elevated circulating levels of arginine vasopressin, the term syndrome of inappropriate antidiuresis (SIAD) is a more accurate description of this condition . Syndrome of Inappropriate Antidiuresis (SIAD) Type C Figure 1 Osmoregulation of plasma arginine vasopressin(AVP) in patients with the syndrome of inappropriate antidiuresis is depicted for types A, B, C, and D. 1 mEq/L = 1 mmol/L. Type A Type B Type D Am J Med 2006; 119: S36-S42 Causes of SIAD N Engl J Med 2007;356:2064-2072 Diagnosis of SIAD N Engl J Med 2007;356:2064-2072 Treatment of SIAD N Engl J Med 2007;356:2064-2072 Vasopressin-Receptor Antagonists Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, 2007 Vasopressin-Receptor Antagonists Ellison D and Berl T. N Engl J Med 2007;356:2064-2072 Demographic and Baseline Characteristics of Patients in the SALT-1 and SALT-2 Trials N Engl J Med 2006;355:2099-2112 Change in the Average Daily Area under the Curve (AUC) for the Serum Sodium Concentration from Baseline to Day 4 (Panel A) and from Baseline to Day 30 (Panel B) The increase in AUC for the serum [Na+] was significantly greater in the tolvaptan group than in the placebo group from baseline to Day 4 as well as during the entire 30-day study period P<0.001 for all comparisons Mild hyponatremia = 130-134 mmol/L Marked hyponatremia < 130 mmol/L N Engl J Med 2006;355:2099-2112 Mean Serum Sodium Concentrations According to the Day of Patient Visit Note: during the week after discontinuation of tolvaptan on day 30, hyponatremia recurred Asterisks indicate P<0.001 for the comparison between tolvaptan and placebo. Daggers indicate P<0.01 for the comparison between tolvaptan and placebo. Tolvaptan was discontinued on day 30. Circles denote patients receiving tolvaptan, and squares denote patients receiving placebo. Horizontal lines indicate the lower limit of the normal range for the serum sodium concentration. Vertical lines indicate the end of the treatment period. HN denotes hyponatremia. N Engl J Med 2006;355:2099-2112 EVEREST Trial: Baseline Participant Characteristics Effects of Oral Tolvaptan in Patients Hospitalized for Worsening Heart Failure: The EVEREST Outcome Trial. Konstam, Marvin; Gheorghiade, Mihai; Burnett, John; Grinfeld, Liliana; Maggioni, Aldo; Swedberg, Karl; Udelson, James; Zannad, Faiez; Cook, Thomas; Ouyang, John; Zimmer, Christopher; Orlandi, Cesare JAMA. 297(12):1319-1331, March 28, 2007. 3 Tolvaptan had no effect on all-cause mortality or the combined end point of cardiovascular mortality or hospitalization for worsening heart failure Effects of Oral Tolvaptan in Patients Hospitalized for Worsening Heart Failure: The EVEREST Outcome Trial. Konstam, Marvin; Gheorghiade, Mihai; Burnett, John; Grinfeld, Liliana; Maggioni, Aldo; Swedberg, Karl; Udelson, James; Zannad, Faiez; Cook, Thomas; Ouyang, John; Zimmer, Christopher; Orlandi, Cesare JAMA. 297(12):1319-1331, March 28, 2007. 4 Tolvaptan had no effect on all-cause mortality or the combined end point of cardiovascular mortality or hospitalization for worsening heart failure Kaplan-Meier Analyses of All-Cause Mortality and Cardiovascular Mortality or Hospitalization for Heart Failure Effects of Oral Tolvaptan in Patients Hospitalized for Worsening Heart Failure: The EVEREST Outcome Trial. Konstam, Marvin; Gheorghiade, Mihai; Burnett, John; Grinfeld, Liliana; Maggioni, Aldo; Swedberg, Karl; Udelson, James; Zannad, Faiez; Cook, Thomas; Ouyang, John; Zimmer, Christopher; Orlandi, Cesare JAMA. 297(12):1319-1331, March 28, 2007. 5


![Effective osmolality: 2 x ([Na] + [K])](http://s3.studylib.net/store/data/008663387_1-590320e85e31378083d542e43b1837df-300x300.png)