Ideal Gas Processes
advertisement

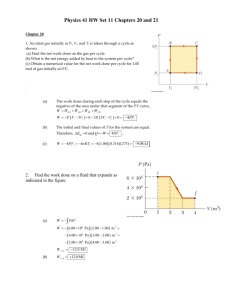
First Law of Thermodynamics Eint Q W Qin Won Heat ADDED to system: Heat REMOVED from system: Qin 0 Qin 0 Qout Qin Qin Eint Work done ON system BY environment: Won 0 Work done BY system ON environment: Won 0 Won Wby Won ADD heat to system or do work ON system: REMOVE heat from system or work done BY system: INCREASE internal energy DECREASE internal energy Eint 0 Eint 0 Work W F ds W 0 to W 0 Force PARALLEL to displacement: Force OPPOSITE displacement: Wby ds Fby gas Fby ds P Ads Ads dV Wby ds Fby gas Volume INCREASE Volume DECREASE Wby 0 Wby 0 Won 0 Won 0 PdV Won Wby PdV (based on 20.27) The figure shows two processes, A and B, carried out on an ideal gas. Consider the following choices: 1. A 2. B 3. Same 4. Not enough information a) In which process was the amount of work done by the gas greater? b) In which process was the change in energy of the gas greater? c) In which process was the energy transferred thermally to the gas greater? Using a P-V diagram P, kPA Consider a monatomic ideal gas, that begins at an initial pressure and volume of 200 kPa and 0.01 m3 . The pressure and the volume of the gas are reduced as shown in the P-V diagram, to a final pressure and volume of 100 kPa and 0.005 m3. What are Won, Qin, and Eint for this process? Wby PdV Won Wby 200 100 .005 0.01 V, m3 Area under curve = Area of rectangle + Area of Triangle = –0.75 kJ = +0.75 kJ Eint Eint, f Eint,i 32 NkT f 32 NkTi 23 Pf V f 23 PiVi Don’t forget the First Law! Eint Qin Won Qin Eint Won = –2.25 kJ – (+0.75 kJ) = –3.0 kJ = –2.25 kJ Was heat added or removed during this process? (monatomic) Constant Volume Process Ideal Gas Processes – Special Case PV NkT P Eint 32 NkT f 32 NkTi 32 ( Pf V f PiVi ) V 32 ( Pf Pi )V P increase Pf Pi T f Ti Eint increase Eint 0 P decrease Pf Pi T f Ti Eint decrease Eint 0 Wby PdV Area under curve Won 0 Wby 0 Constant Volume Process NO WORK DONE Eint Qin Won Qin Eint Won Qin Eint Constant Pressure Process (monatomic) PV NkT P Eint 32 NkT f 32 NkTi 32 ( Pf V f PiVi ) V 32 P (V f Vi ) Ideal Gas Processes – Special Case V increase V f Vi T f Ti Eint increase Eint 0 V decrease V f Vi T f Ti Eint decrease Eint 0 Wby PdV Vf P dV P(V f Vi ) Vi Won Wby Eint Qin Won Qin Eint Won Constant Temperature Process Iso (monatomic) Thermal P Vf Ideal Gas Processes – Special Case Vf NkT Wby PdV dV V Vi Vi Vf dV NkT NkT ln V Vi Vi Vf V PV NkT NkT cons. P V V Eint 32 NkT f 32 NkTi 0 Isothermal Process NO CHANGE IN INTERNAL ENERGY Vf PiVi ln Vi Vf Pf V f ln Vi Won Wby Eint Qin Won Qin Won Isentropic (Adiabatic) Process PV NkT (monatomic) Ideal Gas Processes – Special Case P V cons. monatomic cons. P V Pi Vi Pf V f Qin 0 5 3 Consider adiabatic expansion of gas: V increase Wby 0 Won 0 Won 0 Eint 0 T decrease P Isotherm (high T) Eint NkT f NkTi 3 2 3 2 32 ( Pf V f PiVi ) Eint Qin Won Won Eint Isotherm (low T) Adiabat Vi Vf Adiabat (Isentrope) STEEPER than Isotherm (through same point) V (based on 20.58) A sample of diatomic gas (d = 5, = 5/3) begins in the state 1 shown on the PV diagram and ends in the state 3. Determine everything you can about states, changes in states, and processes.