Kinetic Theory of Ideal Gases
advertisement
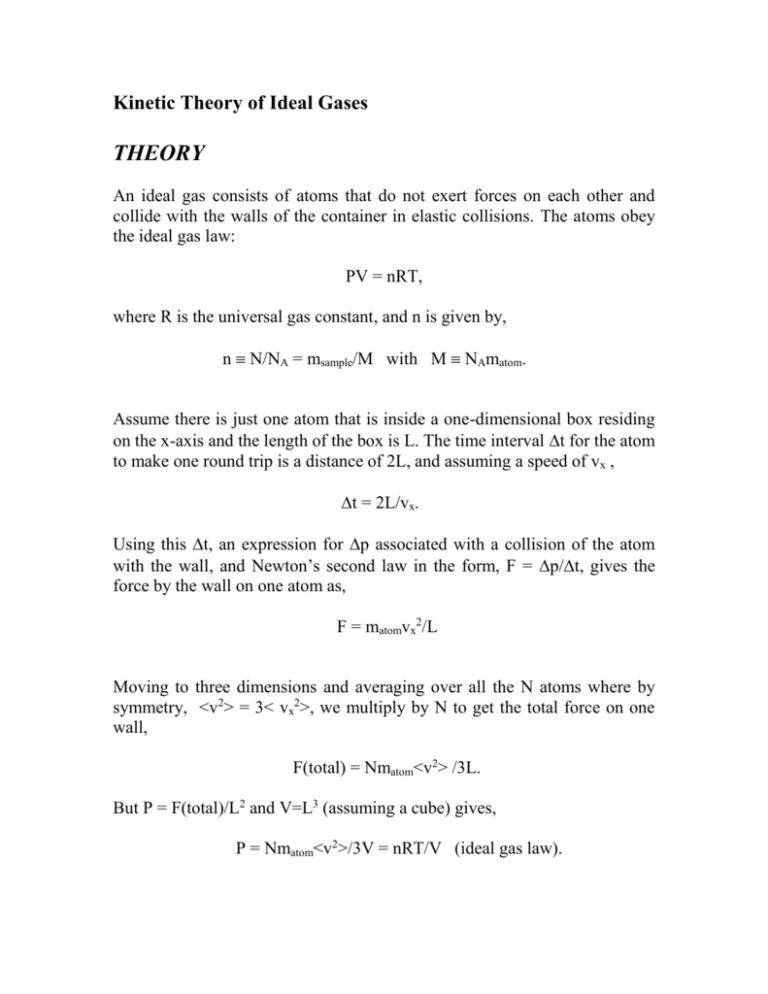
Kinetic Theory of Ideal Gases THEORY An ideal gas consists of atoms that do not exert forces on each other and collide with the walls of the container in elastic collisions. The atoms obey the ideal gas law: PV = nRT, where R is the universal gas constant, and n is given by, n N/NA = msample/M with M NAmatom. Assume there is just one atom that is inside a one-dimensional box residing on the x-axis and the length of the box is L. The time interval t for the atom to make one round trip is a distance of 2L, and assuming a speed of vx , t = 2L/vx. Using this t, an expression for p associated with a collision of the atom with the wall, and Newton’s second law in the form, F = p/t, gives the force by the wall on one atom as, F = matomvx2/L Moving to three dimensions and averaging over all the N atoms where by symmetry, <v2> = 3< vx2>, we multiply by N to get the total force on one wall, F(total) = Nmatom<v2> /3L. But P = F(total)/L2 and V=L3 (assuming a cube) gives, P = Nmatom<v2>/3V = nRT/V (ideal gas law). Using n = N/NA and R = NAkB leads to, matom<v2>/3V = kBT or Eintone atom = ½matom<v2> = 3/2 kBT. If there are N atoms or n = N/NA moles, this becomes, Eint = 3/2 nRT (for a monatomic ideal gas = “m.i.g.”) In summary, (i) (ii) (iii) (iv) the internal energy of an ideal gas depends only on its absolute temperature; temperature is a measure of the random kinetic energy of atoms; this equation is remarkable since it provides a connection between the macroscopic world (n, T) and the microscopic world (Eint of a gas of atoms), and finally this results conforms to equipartition theorem where Eint = no. degrees of freedom x ½ kBT. Since the internal energy of a m.i.g. is entirely kinetic we have Eint = ½ msample <v2> = 3/2 n R T, which gives the root mean square speed of an atom of the gas vRMS = [ 3RT/M]1/2 . Caution: Keep straight n, N, NA, matom, msample, and M. These are six distinct quantities. EXAMPLES [in class]