Part I
advertisement

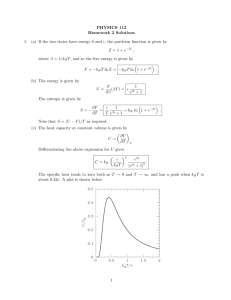
Partition Functions for Independent Particles Independent Particles • We now consider the partition function for independent particles, i.e., particles that do not in any way interact or associate with other molecules • We consider two cases: distinguishable and indistinguishable Distinguishable Particles • Particles that can be differentiated from each other • The particles could be in some way labeled (e.g. red vs. blue) or kept at a fixed position (e.g. particles in a crystal lattice) Indistinguishable Particles • Particles that cannot be differentiated from each other • These particles can interchange locations, so you cannot tell which particle is which (e.g. gas particles) Partition Functions for Independent Particles Model System • Consider a system with energy levels Ej • The system consists of two independent subsystems with energy levels ei and em Distinguishable Particles • Label the two systems A and B • The system energy is E j e iA e mB • Where, i = 1,2,…,a and m = 1,2,…,b • The partition function of each subsystem is a qA e i 1 e iA b kT and qB e m1 e mB kT Partition Functions for Independent Particles Distinguishable Particles • The partition function for the entire system is t Q e i 1 E j kT a b e e iA e mB kT i 1 m1 a b e e iA kT e mB kT e i 1 m1 • Because the subsystems are independent and distinguishable by their labels, the sum over i levels of A has nothing to do with the sum over m levels of B • Therefore, the partition function can be factored a Q e i 1 e iA b kT e e mB kT m1 q A qB • More generally, when we have N independent and distinguishable particles the partition function simplifies to Q qN Partition Functions for Independent Particles Indistinguishable Particles • There are no labels A or B the particles from each other • The system energy is E j ei e m • Where, i = 1,2,…,t1 and m = 1,2,…,t2 • The system partition function is t Q e i 1 E j kT t1 t2 e e i e m kT i 1 m1 • The summation can no longer be separated • As a result of performing the full summation, you overcount by a factor of 2! • Therefore, for N indistinguishable particles, the partition function evaluates to qN Q N! Ideal Gases Molecular Definition • For an ideal gas there are no intermolecular interactions, i.e., F = 0 • The molecules are unaware of each other’s existence and behave independently – the state molecule j is in is completely unaffected by the state of the other molecules in the gas Molecular Energies • We write the total energy of each molecule as the sum of the translational (kinetic) and internal energies e e trans eint • The internal energies include rotational, vibrational, electronic, and nuclear energies eint e rot e vib e elect e nucl Ideal Gases Molecular Energies • The schematic below gives an indication of the relative spacing between energy levels for the various energies electronic vibrational rotational translational Energy Levels Spectroscopy • Spectroscopy measures the frequency n of electromagnetic radiation that is absorbed by an atom, a molecule, or a material • Adsorption of radiation by matter leads to an increase in its energy by an amount e hn • This change is the difference from one energy level to another on an energy ladder Rotational (l) and vibrational (n) energy levels for HBr Ideal Monatomic Gas Monatomic Gas • A monatomic gas has translational, electronic, and nuclear degrees of freedom • The translational Hamiltonian is separable from the electronic and nuclear degrees of freedom • The electronic and nuclear Hamiltonians are separable to a very good approximation • The molecular partition function can be written as qV , T qtrans qelect qnucl • Each factor is treated independently – we now look at the various contributions Energy Levels Quantum Mechanics • The basis for predicting quantum mechanical energy levels is the Schrödinger equation Hy E jy • The wavefunction y(x,y,z) is a function of spatial position • The square of this function, y2, is the spatial probability distribution of the particles for the problem of interest • The Hamiltonian operator H describes the forces relevant for the problem of interest • In classical mechanics the Hamiltonian is given by the sum of kinetic and potential V energies • For example, for the one-dimensional translational motion of a particle having mass m and momentum p p2 H V x 2m Energy Levels Quantum Mechanics • While classical mechanics regards p and V as functions of time and spatial position, quantum mechanics regards p and V as mathematical operators that create the right differential equation for the problem at hand • In one dimension, the translational momentum operator is 2 2 h d p2 2 2 4 dx • Schrödinger’s equation is now h 2 d 2y x 2 V x y x E jy x 2 8 m dx • Only certain functions y(x) satisfy this equation • The quantities Ej are called eigenvalues and represent the discrete energy levels that we seek • The index j for the eigenvalues is called the quantum number Translational Motion The Particle-in-a-Box Model • The particle-in-a-box is a model for the freedom of a particle to move within a confined space • A particle is free to move along the x-axis over the range 0 < x < L • At the walls (x = 0 and x = L) the potential is infinite (V(0) = V(L) = ∞) • Inside the box the molecule has free motion (V(x) = 0 for 0 < x < L) • Schrödinger’s equation is d 2y 2 K y 0 2 dx with 2 8π me 2 K h2 • The solution to this differential equation is y x A sin Kx B cos Kx Translational Motion The Particle-in-a-Box Model • The boundary conditions are y 0 0 and • Applying the boundary conditions and normalizing the probability distribution gives the following expressions for the wavefunction yn and energy en for a given quantum number n 2 n x y n x sin 12 L n2 h2 en 8mL2 L n 1, 2,3, y L 0 Ideal Monatomic Gas Translational Partition Function • Quantum mechanics (particle in a box) tells us that the translational energy levels for a particle in a three-dimensional box are given by h2 2 2 2 n n n y z 2 x 8mL en n n x y z nx , ny , nz 1, 2, • The translational molecular partition function is now given by qtrans e e nx n y nz nx ,n y ,nz 1 e h 2 nx2 8 mL2 e nx 1 n y 1 n t T e n1 2 3 h 2n 2y 8 mL2 e nz 1 h 2nz2 8 mL2 h2 t 8mL2 k Example: For argon in a 1 cm3 box, t 6.0 1016 K Ideal Monatomic Gas Translational Partition Function • At most temperatures of practical importance, the energy levels are narrowly spaced in comparison to kT • Therefore, we can replace the sum with an integral qtrans e 0 h2n2 8 mL2 dn 3 • which evaluates to 2 mkT qtrans V , T V 2 h 32 • Using the de Broglie wavelength L, the result is qtrans V 3 L with 12 h L 2 mkT 2 Ideal Monatomic Gas Electronic Partition Function • The electronic partition function is usually written as a sum over energy levels rather than a sum over states qelect wei e ei • Where wei and ei are the degeneracy and energy of the ith electronic level respectively • Generally, the first electronic level is set as the ground state (e1 = 0) and the remaining energy levels are expressed in terms of their spacing relative to that of the ground state qelect we1 we 2e e12 • Where e1j is the energy of the jth electronic level relative to the ground state • At ordinary temperatures, usually only the ground state and perhaps the first excited state need to be considered Ideal Monatomic Gas Electronic Partition Function • Here are the electronic energies for a number of atoms Ideal Monatomic Gas Example 1. What fraction of helium atoms are in their first excited state at T = 300 K and T = 3000 K 2. What fraction of fluorine atoms are in their first excited state at T = 200, 400, and 1000 K Ideal Monatomic Gas Nuclear Partition Function • The nuclear partition function has a form similar to that of the electronic partition function • Nuclear energy levels are separated by millions of electron volts, which means temperatures on the order of 1010 K need to be reached before the first excited state becomes populated to any significant degree • Therefore, at ordinary temperatures we can simply express the nuclear partition function in terms of the degeneracy of the ground state qnucl wn1 • With this simplification the nuclear partition function only contributes to entropies and free energies Ideal Diatomic Gas Overview • To a high degree of accuracy diatomic molecules can be described using the rigid rotor – harmonic oscillator approximation • With this approximation, in addition to the translational, electronic, and nuclear energies, the molecule has two additional energies • Rotational (rigid rotor): rotary motion about the center of mass • Vibrational (harmonic oscillator): relative vibratory motion of the two atoms • Again, we assume that all energies are independent, which gives the following expression for the molecular partition function q qtrans qrot qvib qelect qnucl Ideal Diatomic Gas Energy levels • A quantum mechanical solution to the rigid rotor – harmonic oscillator problem gives the following energy levels and degeneracies for the rotational and vibrational motion Rigid Rotor h 2 J J 1 eJ 8 I Harmonic Oscillator J 0,1, 2, I re2 re → bond length → reduced mass n 0,1,2, wn 1 wJ 2 J 1 I → moment of inertia e n hv n 12 m1m2 m1 m2 2 B → rotational const. B h 8 Ic 12 v → frequency 1 k v 2 k → force constant w → wave number w v c Ideal Diatomic Gas Ground State • Before obtaining the partition function, we need to set the ground state • We take the rotational ground state as the J = 0 state De Do 12 hn • For the vibrational energy, we have Do 12 k v two choices Take the lowest vibrational state to be the ground state Take the bottom of the interatomic potential well as zero energy • We adopt the second choice • We take the zero of the electronic energy to be the separated, electronically unexcited atoms at rest • The electronic partition function is now qelect we1e De we 2e e 2 Ideal Diatomic Gas Vibrational Partition Function • With our choice of the ground state as the minimum in the interatomic potential well, the vibrational energies are given as e n n 12 hv n 0,1,2, • The partition function is given by a sum over all energy levels qvib T e e n n e hv 2 e hvn n 0 • This summation can be evaluated analytically nx e n 0 1 1 e x Ideal Diatomic Gas Vibrational Partition Function • Performing the summation gives the following exact solution e hv 2 qvib T 1 e hv • The vibrational temperature, v ≡ hv/k, is often used instead of the vibrational frequencies ev 2T qvib T 1 e v T Ideal Diatomic Gas Vibrational Partition Function • Thermodynamic properties are found in the usual way U vib v ln qvib v NkT Nk v T 1 T 2 e 2 v T U e vib k v 2 T T ev T 1 2 Cv, vib • Here is a plot of the vibrational contribution to the heat capacity as a function of temperature Ideal Diatomic Gas Rotational Partition Function • The rotational energy levels are h 2 J J 1 eJ 8 I wJ 2 J 1 J 0,1, 2, • The partition function is given by a sum over all energy levels qrot T 2 J 1 e BJ J 1 J 0 • This summation cannot be written in closed form • At ordinary temperatures the energy levels are usually narrowly spaced compared to kT and we can approximate the sum by an integral qrot T 0 2 J 1 e J J 1 T dJ r • Where we have introduced the rotational temperature r B k Ideal Diatomic Gas Rotational Partition Function • The integral evaluates to T qrot T r • This approximate solution is valid for temperatures that far exceed the characteristic temperature for rotation, i.e., T >> r • Replacing the rotational temperature with the moment of inertia gives 8 2 IkT qrot T h2 • As T approaches r the approximate solution is no longer valid and the actual summation must be performed Ideal Diatomic Gas Rotational Partition Function • The approximate solution gives the following values for the energy and heat capacity U rot NkT Cv, rot k Ideal Diatomic Gas Molecular Constants • Here are the molecular constants for several diatomic molecules Ideal Diatomic Gas Symmetry Number • The symmetry number (s) is a classical correction factor introduced to avoid over counting indistinguishable configurations • Its origin is quantum mechanical in nature and the approach presented here is appropriate at ordinary temperatures • The symmetry number represents the number of different ways a molecule can be rotated into a configuration indistinguishable from the original • By accounting for the symmetry of a molecule, the rotational partition function becomes T qrot T s r Ideal Diatomic Gas Symmetry Number • Here are the symmetry numbers for a number of common molecules Formula HCl N2 s 1 2 Carbonyl Sulfide Carbon Dioxide Water Ammonia COS CO2 H2O NH3 1 2 2 3 Methane Benzene CH4 C6H6 12 12 Molecule Hydrogen Chloride Nitrogen Ideal Polyatomic Gas Overview • We now extend the methods we have developed for diatomic molecules to polyatomic systems • We use an analogous approach to that adopted previously for the translational, electronic, and nuclear energies • The vibrational partition function is a straightforward extension of the diatomic case • The rotational partition function is a bit more complicated, however a convenient solution exists as long as we can treat the molecule classically • A new feature that arises with polyatomic molecules is hindered (or internal) rotation (e.g., rotation about the carbon-carbon bond in ethane) • The molecular partition function is q qtrans qrot qint rot qvib qelect qnucl Ideal Polyatomic Gas Translational Partition Function • Once again, the translational partition function is expressed in terms of the de Broglie wavelength qtrans V 3 L with h2 L 2 mkT • Where m is the molecular mass of the molecule Electronic Partition Function • Again, we choose as the zero of energy all n atoms completely separated in their ground electronic states • Therefore, the energy of the ground electronic state is –De • It follows that the electronic partition function is qelect we1e De we 2e e 2 Ideal Polyatomic Gas Vibrational Partition Function • The number of vibrational degrees of freedom a a molecule possesses is the difference between its total number of degrees of freedom (3n) and the sum of the translational (3) and rotational (2 for linear and 3 for nonlinear molecules) degrees of freedom 3n 5 a 3n 6 linear molecule nonlinear molecule • A coordinate transformation can be completed such that a set of coordinates (normal coordinates) are found in which the Hamiltonian can be written in terms of a sum of a independent harmonic oscillators • The total vibrational energy is given by a e n j 12 hv j j 1 n j 0,1, 2, Ideal Polyatomic Gas Vibrational Partition Function • Each of the vibrational modes can be treated independently and the vibrational partition function becomes a qvib j 1 e vj 2T 1 e vj T • It follows that the internal energy and heat capacity are given as U vib vj vj Nk vj T e 1 j 1 2 Cv, vib 2 vj T e k vj T j 1 T e vj 1 2 a a Ideal Polyatomic Gas Vibrational Partition Function • Here’s a look at the vibrational modes for two common molecules symmetric stretch asymmetric stretch bending mode (doubly degenerate) Ideal Polyatomic Gas Rotational Partition Function (Linear Molecules) • For linear molecules, the quantum states are the same as for a diatomic molecule h 2 J J 1 eJ 8 I J 0,1, 2, wJ 2 J 1 • Using the classical approximation the partition function is given by 8 2 IkT T qrot 2 sh sr Ideal Polyatomic Gas Rotational Partition Function (Nonlinear Molecules) • We describe the geometry of a nonlinear molecule in terms of its principal moments of inertia • These values are found with respect to a molecules principal axes, set such that all products of inertia are zero I xy I xz I yz 0 • The principal moments of inertia are usually denoted as follows I xx I A I yy I B I zz I C • The three principal moments of inertia are used to define the corresponding rotational temperatures A, B, and C Ideal Polyatomic Gas Rotational Partition Function (Nonlinear Molecules) • The quantum mechanical energy levels depend on the relative values of the principal moments of inertia • Three cases exist: Spherical Top: IA = IB = IC h 2 J J 1 eJ 8 I IA = IB ≠ IC Symmetric Top: e JK wJ 2 J 1 J 0,1, 2, h 2 J J 1 1 2 1 K 8 I A IC I A Asymmetric Top: J 0,1, 2, K J , J 1, , J IA ≠ I B ≠ IC A closed form solution does not exist wJK 2 J 1 Ideal Polyatomic Gas Rotational Partition Function (Nonlinear Molecules) • If T is close to A, B, or C, then the actual summations must be performed to obtain the partition function (the asymmetric top case requires a numerical solution) • In the classical limit T >> a general solution for the rotational partition function can be obtained 12 12 12 8 I A kT 8 I B kT 8 I C kT qrot s h 2 h 2 h 2 12 2 2 2 12 T qrot s A B C 12 3 • Simple relationships result for the internal energy and heat capacity Cv, rot 32 k U rot 32 NkT Ideal Polyatomic Gas Molecular Constants • Here are the molecular constants for several polyatomic molecules • For a polyatomic molecule Do and De are related by a Do De 12 hv j j 1 Ideal Polyatomic Gas Hindered Rotation • Consider the rotation about the carbon-carbon bond in ethane • The potential energy as a function of the angle f is as follows • The treatment of this problem depends upon the value of Vo relative to that of kT • Let’s take a look at three possibilities Ideal Polyatomic Gas Hindered Rotation • kT >> Vo: in this case the internal rotation is essentially free and can be treated by methods similar to that for the rigid rotor • kT << Vo: in this case the molecule is trapped at the bottom of the wells and the motion is that of a simple torsional vibration, which can be treated by a method similar to that used for the simple harmonic oscillator • kT ≈ Vo: in this case neither of the above approximations is valid and the full quantum mechanical problem must be solved – the solutions have been tabulated for many common molecules • Unfortunately, for many molecules of practical significance, the latter case is found