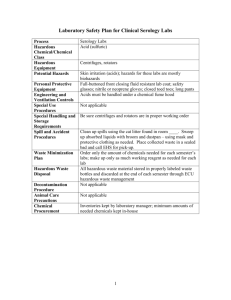
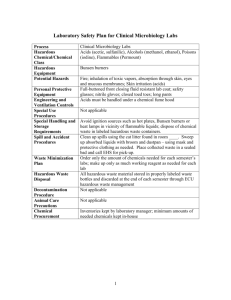
Kristin Fitzgerald
advertisement

1 Part I: Academic Labs Rule (Subpart K) › Finalized in 2008 Part II: HW Pharmaceuticals Proposed Rule › Under development Questions 2 1. Overview of Academic Labs Rule 2. 6 Main Features 3. Where is the Labs Rule in Effect? 4. Who is using the Labs Rule? 5. 3 Mythbusters 3 Academia’s Complaint: RCRA generator rules are not a good fit for academic laboratories › › › › Lots of different wastes that vary over time Small amounts of each waste Lots of points of generation Wastes generated by students who have high turnover and lack accountability EPA’s Response: a sector-based RCRA generator rule for Academic Laboratories to be used in lieu of satellite accumulation area regulations › Part 262 Subpart K 4 Only “Eligible Academic Entities” may use the Labs Rule: › Colleges & Universities (C/Us) › Teaching Hospitals affiliated with C/Us › Non-profit Research Institutes affiliated with C/Us Any size generator may use the Labs Rule › CESQGs › SQGs › LQGs Other labs may not use the Labs Rule: › Government R&D labs › Commercial R&D labs 5 The Labs Rule is optional on two levels: › States have the option of adopting the rule › Eligible Academic Entities have the option of using the Labs Rule in lieu of satellite accumulation area regulations Eligible Academic Entities can not opt into using the Labs Rule unless their state has adopted it 6 Under RCRA, HW Determinations have to be made at the point of generation Students and PIs that generate the waste in labs lack the expertise needed to make HW determinations The Academic Labs Rule gives the responsibility for making HW determinations to the EH&S professionals Lab personnel must give anything that has the potential to be a hazardous waste to EH&S for a HW determination › Labeling is required to allow EH&S to make proper HW determination 7 Labs Rule requires hazardous waste to be removed from labs based on time or volume › Every 6 months, regardless of volume › Kept volume limits as a back-up: More than 55 gallons HW More than 1 quart of acute HW 8 Labs Rule allows each lab to do one clean-out per year (not mandatory) › Hazardous waste from lab clean-outs do not count toward generator status › Hazardous waste from lab clean-outs must be managed as hazardous waste › 30 days to conduct a lab cleanout › This addresses the unintended disincentives of the current program for cleaning out old chemicals 9 Each eligible academic entity that opts in, must write an LMP with 9 elements: › Part I – 2 elements (enforceable) Identify choices that the rule requires › Part II – 7 elements (not enforceable) Describe processes and procedures for how the labs and the EH&S will communicate and manage the laboratory hazardous waste 10 11 1. Georgia 2. Kentucky 3. Massachusetts 4. Nevada 5. New York 6. Oregon 7. South Carolina 8. Tennessee 9. Texas 10. Washington 12 61 Academic Entities have opted to use Subpart K, including › Colleges/Universities › Non-profit Research Institutes › Teaching Hospitals 13 MYTH #1: › The Labs Rule is only good for small schools REALITY: › Nearly 50% of the those that have opted in are LQGs 14 MYTH #2: › If you opt into the Labs Rule, you have to comply with more than one RCRA on campus REALITY: › As EH&S, you probably already do – Used Oil, Universal Waste, etc. › Your lab workers only have to comply with one RCRA 15 MYTH #3: › EPA should have made the rule apply to the entire campus REALITY: › Other areas of campus do not share the same issues that labs have, so we had no basis for extending the applicability beyond labs 16 http://www.epa.gov/multimedia/ORCR/index.html 17 1. Update on Proposed Rule 2. Wiki for healthcare professionals to share info on which pharmaceuticals are hazardous waste 3. Mythbusters 18 EPA proposed to add hazardous waste pharmaceuticals to the Universal Waste program in December 2008 Commenters expressed concern over: › Lack of notification › Lack of tracking/security EPA decided we could not finalize as proposed and address the commenters’ concerns EPA is moving forward with a new proposal for sector-specific regulations for the management of hazardous waste pharmaceuticals 19 Sector-based rulemaking for healthcare facilities and reverse distributors EPA is building on the Universal Waste proposal Our approach has been: › Keep aspects of the UW proposal that commenters liked › Address commenters’ concerns › Address new areas that the UW proposal did not 20 Episodic generation due to P-listed hazardous waste Residues in containers that once held Plisted pharmaceuticals Flushing pharmaceuticals Uncertain regulatory status of reverse distributors Intersection of DEA & EPA regulations 21 DEA published a proposed rule to provide disposal options for ultimate users (consumers) of controlled substances on December 21, 2012 › EPA commented on the proposal during interagency review There are a few hazardous wastes that are also controlled substances EPA is coordinating with DEA to develop a workable solution 22 Healthcare facilities often struggle with determining which drugs in its formulary of thousands of drugs are hazardous waste EPA created a platform for the healthcare sector to share its expertise with other members of the healthcare sector on which drugs are hazardous wastes – like wikipedia Anyone can view the material in the wiki Upon request/approved registration, members of the healthcare community, regulators, etc., can contribute and/or edit the material in the wiki The wiki is fully searchable by drug name, brand name, waste code, etc 23 24 MYTH: › The new proposed rule will regulate ALL waste pharmaceuticals as hazardous waste REALITY: › If EPA adds pharmaceuticals to the hazardous waste listings or characteristics, that will be a separate rulemaking in the future 25 Kristin Fitzgerald › 703-308-8286 › Fitzgerald.Kristin@epa.gov Academic Labs http://www.epa.gov/waste/hazard/generation/labwaste Pharmaceuticals http://www.epa.gov/epawaste/hazard/generation/ pharmaceuticals.htm 26