changes in matter
advertisement

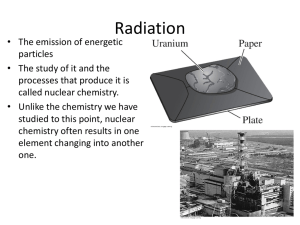
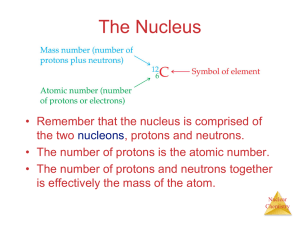
CHANGES IN MATTER 1) USING the RECYCLED PAPER, CREATE A SYMBOL THAT WILL REPRESENT YOUR GROUP FOR THIS QUARTER. 2) YOUR SYMBOL SHOULD HAVE A RELATION WITH CHEMISTRY. 3) YOU ARE ONLY GIVEN 10 MINUTES TO CREATE YOUR SYMBOL. 4) YOU WILL BE GRADED ACCORDING TO THE FOLLOWING: a) Creativity - 5 b) Originality- 5 c) Compliance - 5 d) Content – 10 TOTAL : 25 pts. TO DETERMINE IF CHANGE IS PHYSICAL OR CHEMICAL, ANSWER THE FOLLOWING QUESTIONS: 1)Do you still have the same object you started with? 2) Can it be changed back to its original form or is it reversible? If both YES – PHYSICAL change has occurred. If a NO is answered in any of the questions – CHEMICAL change has occurred. PHYSICAL CHANGE is a reversible change that affects the physical property of a substance but NOT its composition. CHEMICAL CHANGE is an irreversible change that alters the composition of a substance. + Na REACTANT (original substance) Cl REACTANT (original substance) NaCl PRODUCT (new substance) The reaction is ALWAYS accompanied by ENERGY change. (heat, light and electrical) Chemical change has occurred if one can see these evidences or signs during the reaction. evolution of gas Evolution of heat and light Chemical change has occurred if one can see these evidences or signs during the reaction. Formation of a precipitate (insoluble substance) Chemical change has occurred if one can see these evidences or signs during the reaction. Production of Mechanical energy Production of Electrical energy NUCLEAR CHANGE is the alteration in the nucleus of the atom and may change the identity of the atom. Natural Radioactivity Artificial Radioactivity NUCLEAR CHANGE is the alteration in the nucleus of the atom and may change the identity of the atom. Nuclear Fusion (combining) Nuclear Fission (splitting) Classify if it’s a Physical or Chemical Change Banana Browning Chemical Salt dissolving in water Physical Tin alloy melts at 505 K Physical Gasoline igniting Chemical Milk souring Chemical Iron bolt rusting Chemical Wine turning to vinegar Chemical Alcohol evaporating Physical Silver table spoon tarnishes Chemical Physical Water turns to vapor Acknowledgements & References Dr. Richard Gross Chemistry Professor Ateneo De Manila University Silberberg,M., Chemistry: The Molecular Nature of Matter & Change. McGraw Hill International 2007 Chang, R. Chemistry. 7th edition. McGraw Hill International 2002