NT Ch 4 & 25 word
advertisement
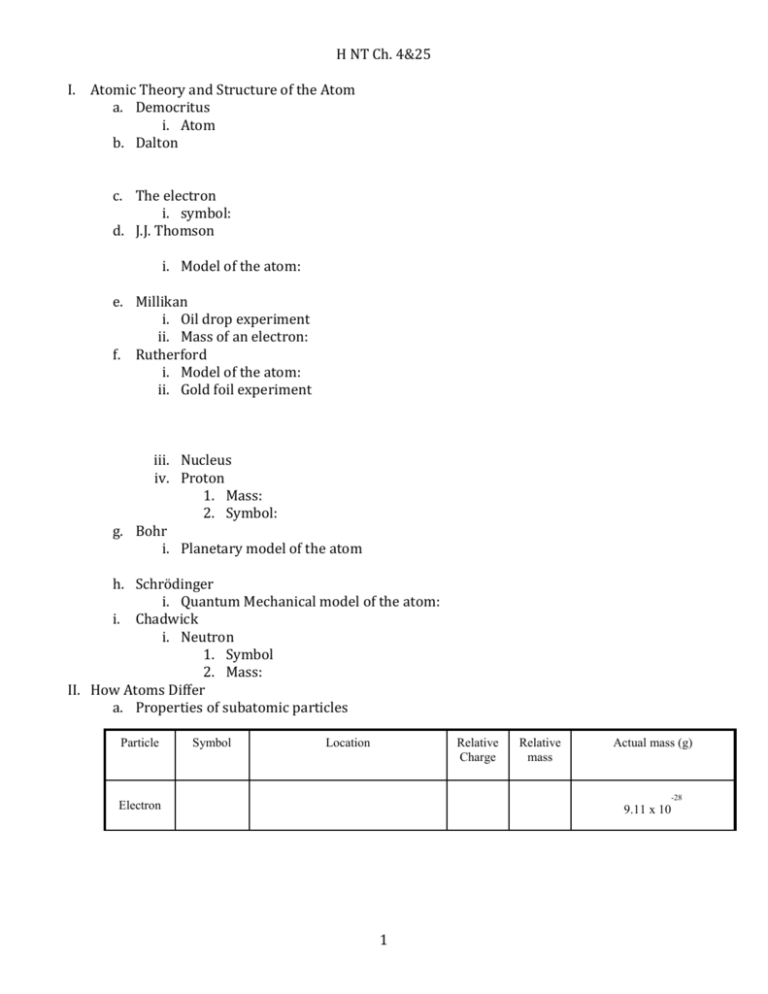
H NT Ch. 4&25 I. Atomic Theory and Structure of the Atom a. Democritus i. Atom b. Dalton c. The electron i. symbol: d. J.J. Thomson i. Model of the atom: e. Millikan i. Oil drop experiment ii. Mass of an electron: f. Rutherford i. Model of the atom: ii. Gold foil experiment iii. Nucleus iv. Proton 1. Mass: 2. Symbol: g. Bohr i. Planetary model of the atom h. Schrödinger i. Quantum Mechanical model of the atom: i. Chadwick i. Neutron 1. Symbol 2. Mass: II. How Atoms Differ a. Properties of subatomic particles Particle Symbol Location Relative Charge Relative mass Actual mass (g) -28 Electron 9.11 x 10 1 -24 Proton 1.673 x 10 Neutron 1.675 x 10 -24 b. Atomic Number c. Mass number d. Isotopes i. Example e. Average atomic i. Formula: ii. Example 1: Silver has two naturally occurring isotopes. Ag-107 has an abundance of 51.82% and mass of 106.9 amu. Ag-109 has a relative abundance of 48.18% and a mass of 108.9 amu. Calculate the atomic mass of silver. iii. Rubidium is a soft, silvery-white metal that has two common isotopes, 85 37Rb and 87 85 87 37Rb. If the abundance of Rb is 72.2% and the abundance of Rb is 27.8%, what is the average atomic mass of rubidium? III. Nuclear Chemistry a. Chemical vs. nuclear reactions b. Electrostatic force c. Strong Nuclear Force d. Radiation e. Radioactivity i. Alpha 1. Symbol: 2. Charge: ii. Beta 1. Symbol: 2. Charge: 2 iii. Gama 1. Symbol: 2. Charge f. Radioactive i. Transmutation 1. Can be induced (caused) 2. Transuranium elements g. Radioactive decay i. Band of stability h. Radioisotope i. Small nuclei 1. Exception ii. Large nuclei i. Electron Capture i. Example: j. Nuclear Equations i. Example ii. Practice 97 0 1. 𝑍𝑟 → 𝑒+? 40 −1 218 4 2. 𝑃𝑜 → 𝐻𝑒 + ? 84 2 222 4 3. ? → 𝑅𝑛 + 𝐻𝑒 86 2 47 0 𝐶𝑎 → 𝑒+? 20 −1 244 4 5. 𝐶𝑚 → 𝐻𝑒 + ? 96 2 4. k. Half-Life i. Equation: ii. Practice 1. Scientists start with 50.0 g sample of a radioisotope. How much is left after four half-lives? 2. Iron-59 is used in medicine to diagnose blood circulation disorders. The halflife of iron 59 is 44.5 days. How much of a 2.000 mg sample will remain after 133.5 days? 3 iii. Carbon-14 dating Using the graph, about what % of carbon-14 remains after 11, 400 years? IV. Nuclear Fission and Fusion a. Fission i. Chain reaction 1. Critical mass b. Fusion i. Example: c. Nuclear binding energy d. Mass Defect e. Nuclear Reactors f. Nuclear Bombs i. Atomic Bomb ii. Hydrogen Bomb 4