Powerpoint
advertisement

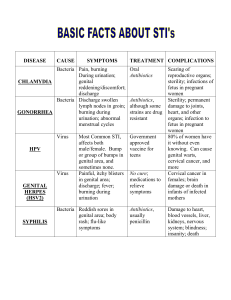
High Discordance in Plasma and Genital Tract HIV-1 Drug Resistance in Indian Women Failing First-line Therapy MOPDA0106 S. Saravanan, PhD Session Code: MOPDA01 Molecular Techniques of HIV-1 Analysis 21 July 2014 Background: Much less work has been directed at HIV in non-blood compartments and those compartments may be the potential sanctuary sites harboring HIV and impacting both the transmission and pathogenesis of HIV infection. It is vital to investigate tissues and compartments other than blood for two important reasons (Cu-Uvin S., et al., 2010). • From a patient perspective, it is important to determine whether antiretroviral therapy can reduce viral load in non-blood compartments. • From a public health perspective, it is critical to know the factors that contribute to the “infectiousness” of an individual in order to devise strategies to reduce the likelihood of transmission. 2 of 10 Materials and Methods: HIV-infected women (n=200) at YRG CARE in Chennai, India, who were adherent on >6 months of first-line antiretroviral therapy were enrolled. Genital tract (2 Sno-strips in 500uL of NASBA buffer) RNA levels measured using COBAS® AMPLICOR HIV-1 MONITOR Test, v1.5 & Pol genotyping (Saravanan et al., 2009) was conducted in paired detectable samples from both compartments. Sequences were aligned (ClustalX) to an Indian subtype C reference (C.IN.AFo67155) and examined for HIV-1 subtype (REGA v2), nucleotide diversity (Highlighter HIV LANL, SLAC in HyPHY) and drug resistance associated mutations (IAS-USA and Stanford HIV Resistance Database). 3 of 10 Results: Demographic details of enrolled study subjects Women on First-line ART (n=200) Patient Characteristics Viremic (n=73) Non-Viremic (n=127) Age(Mean) years 33.8±6.3 33.4±5.2 PVL (Median) log copies/mL 4.6 (3, 5.9) Not Detected CD4(Median) cells/µL 246 (15, 832) 530 (27, 1182) Duration on HAART (Median) Months 35 (7, 114) 34 (6, 122) NVP 44 (60%) 87 (68.5%) D4T/AZT 55 (75%) 114 (90%) 4 of 10 Results: STUDY SUBJECTS ENROLLED; n = 200 VIREMIC; n=73 (36.5%) PVL >3000 Copies/mL; n=42/73 (57.5%) DETECTABLE GVL n=30/42 (71%) GVL >2000 Copies/mL n=21/30 (70%) NON-VIREMIC; n=127 (63.5%) PVL <3000 Copies/mL; n=31/73 (42.5%) UNDETECTABLE GVL n=12/42 (29%) GVL <2000 Copies/mL n=9/30 (30%) 5 of 10 Results: Genital and Plasma Sequences (n = 21) Concordant mutation patterns; n = 4/21 (19%) Discordant mutation patterns; n = 17/21 (81%) 41% 50% Percent of subjects 35% 40% 24% 30% 20% 10% 0% 5 NRTI – T215F, M41L, D67DN, K70T, K219E 7 NNRTI- K103E/N, H221Y, V106M, Y188H, E138A, Y181C Additional in Genital Additional in Plasma Diverse in both Discordance Pattern Total patients with additional mutations in genital tract; n=11/21 (52%) 6 of 10 Results: p= <0.005 r2 = 0.872 (p=ns) Women with detectable PVL tend to shed virus in genital secretions (p<0.005) Figure 1a: Pearson’s rank correlation for comparison between PVL and GVL in patients with discordance (n=17). 1b: Fisher’s exact test for comparison of patients with detectable and undetectable viral load in plasma and genital compartments (n=42) Figure 4: Phylogenetic analyses of RT 95% have monophyletically clustered with Indian subtype C with one sequence clustered to CRF_02 AE 7 of 10 Results: High prevalence of M184V in both compartments followed by TAMs. Figure 5: Prevalence of various NRTI and NNRTI DRMs in plasma and genital tract. Figure 6: Comparison between the presence of intermediate to high-level resistance in plasma and genital secretions in patients with discordant mutations (n=5). 8 of 10 Genital discordant mutations were responsible for an increase to a predicted intermediate or high level drug resistance to at least one drug in 24% of women Conclusion: High resistance discordance between plasma and the genital tract among South-Indian women failing first-line antiretroviral therapy, suggesting compartmentalization and independent viral evolution. If confirmed, GVL and resistance monitoring may need to be considered to prevent sexual and perinatal HIV-1 resistance transmission in countries like India where sexual transmission is the major mode of HIV infection. 9 of 10 Acknowledgement Indian Council of Medical Research (ICMR) under U.S.-India Collaborative Research Supplement # Indo-US/35/2007-ECD-II S. Gomathi, M.Sc S. Sivamalar, M.Sc G. Kausalya, M.Sc P. Selvamuthu, MBBS N. Kumarasamy, MBBS, PhD P. Balakrishnan, PhD Suniti Solomon, MD Susan Cu-Uvin, MD Rami Kantor, MD Sunil S. Solomon, MPH, PhD IAS International scholarship (S12858) 10 of 10