Document
advertisement

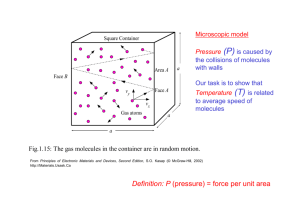
Halliday/Resnick/Walker Fundamentals of Physics 8th edition Classroom Response System Questions Chapter 19 The Kinetic Theory of Gases Reading Quiz Questions 19.1.1. Consider the following variables: (1) mass, (2) temperature, (3) time, (4) length, (5) pressure, (6) volume, and (7) density. Which three variables does the book state are “all a consequence of the motion of the atoms” within a gas? a) 2, 4, and 7 b) 1, 5, and 6 c) 1, 3, and 4 d) 5, 6, and 7 e) 2, 5, and 6 19.1.1. Consider the following variables: (1) mass, (2) temperature, (3) time, (4) length, (5) pressure, (6) volume, and (7) density. Which three variables does the book state are “all a consequence of the motion of the atoms” within a gas? a) 2, 4, and 7 b) 1, 5, and 6 c) 1, 3, and 4 d) 5, 6, and 7 e) 2, 5, and 6 19.2.1. Which one of the following numbers is Avogadro’s number? a) 1.602 1019 mol1 b) 6.022 1023 mol1 c) 4.186 1021 mol1 d) 9.111 1031 mol1 e) 1.6605 1027 mol1 19.2.1. Which one of the following numbers is Avogadro’s number? a) 1.602 1019 mol1 b) 6.022 1023 mol1 c) 4.186 1021 mol1 d) 9.111 1031 mol1 e) 1.6605 1027 mol1 19.2.2. Which one of the following statements concerning the mole is false? a) The mole is related to Avogadro's number. b) The mole is defined in terms of the carbon-12 isotope. c) The mole is the SI base unit for expressing the “amount” of a substance. d) One mole of a substance has the same mass as one mole of any other substance. e) One mole of a substance contains the same number of particles as one mole of any other substance. 19.2.2. Which one of the following statements concerning the mole is false? a) The mole is related to Avogadro's number. b) The mole is defined in terms of the carbon-12 isotope. c) The mole is the SI base unit for expressing the “amount” of a substance. d) One mole of a substance has the same mass as one mole of any other substance. e) One mole of a substance contains the same number of particles as one mole of any other substance. 19.2.3. Which of the following gives the correct order of magnitude of the number of particles in a mole? a) 1018 b) 1020 c) 1021 d) 1023 e) 1026 19.2.3. Which of the following gives the correct order of magnitude of the number of particles in a mole? a) 1018 b) 1020 c) 1021 d) 1023 e) 1026 19.3.1. Which one of the following statements concerning the volume of gases is true? a) Gas volume depends on temperature and pressure. b) Gases have comparatively low densities. c) Gas volume depends on the type of gas. d) Gas volume is negligible. e) Gas volume is difficult to measure. 19.3.1. Which one of the following statements concerning the volume of gases is true? a) Gas volume depends on temperature and pressure. b) Gases have comparatively low densities. c) Gas volume depends on the type of gas. d) Gas volume is negligible. e) Gas volume is difficult to measure. 19.3.2. The volume of a carbon dioxide bubble rising in a glass of beer is observed to nearly double as the bubble rises from the bottom to the top of the glass. Why does the volume nearly double? a) The fluid pressure of the beer is greater at the bottom of the glass than at the top. b) The shape of the glass determines the net force exerted on the bubble. c) The pressure inside the bubble decreases as it rises. d) The temperature at the bottom is cooler than it is at the top. e) The amount of carbon dioxide in the bubble increases. 19.3.2. The volume of a carbon dioxide bubble rising in a glass of beer is observed to nearly double as the bubble rises from the bottom to the top of the glass. Why does the volume nearly double? a) The fluid pressure of the beer is greater at the bottom of the glass than at the top. b) The shape of the glass determines the net force exerted on the bubble. c) The pressure inside the bubble decreases as it rises. d) The temperature at the bottom is cooler than it is at the top. e) The amount of carbon dioxide in the bubble increases. 19.3.3. There are n moles of an ideal gas contained in a sealed chamber at pressure P. The volume of the container is then reduced to one half of its initial value. In this particular process, the temperature of the gas and n do not change. Which one of the following statements concerning the final pressure in the container is true? a) The final pressure will be 2P. b) The final pressure will be 0.5P. c) The final pressure will be 4P. d) The final pressure will be 0.25P. e) The pressure cannot be determined without knowing the values of n, P, T, and the initial volume. 19.3.3. There are n moles of an ideal gas contained in a sealed chamber at pressure P. The volume of the container is then reduced to one half of its initial value. In this particular process, the temperature of the gas and n do not change. Which one of the following statements concerning the final pressure in the container is true? a) The final pressure will be 2P. b) The final pressure will be 0.5P. c) The final pressure will be 4P. d) The final pressure will be 0.25P. e) The pressure cannot be determined without knowing the values of n, P, T, and the initial volume. 19.3.4. There are five sealed containers, each containing the same number of moles of an ideal gas. The pressures and volumes for the five containers are: (1) 2 105 Pa and 0.25 m3, (2) 4 105 Pa and 1.0 m3, (3) 1 105 Pa and 2.0 m3, (4) 6 105 Pa and 0.25 m3, and (5) 4 105 Pa and 0.50 m3, respectively. Which container is at the highest temperature? a) 1 b) 2 c) 3 d) 4 e) 5 19.3.4. There are five sealed containers, each containing the same number of moles of an ideal gas. The pressures and volumes for the five containers are: (1) 2 105 Pa and 0.25 m3, (2) 4 105 Pa and 1.0 m3, (3) 1 105 Pa and 2.0 m3, (4) 6 105 Pa and 0.25 m3, and (5) 4 105 Pa and 0.50 m3, respectively. Which container is at the highest temperature? a) 1 b) 2 c) 3 d) 4 e) 5 19.3.5. A girl uses a pump to put air into her bicycle tire at constant temperature. A pressure gauge on the pump indicates the pressure inside the tire is increasing each time she pumps. What is the cause of this pressure increase? a) The air molecules repel each other more as more molecules are added and push outward. b) The volume of the tire is constant. c) The pressure increases to keep the temperature constant. d) The pressure increasing because more air molecules are striking the walls of the tire. e) The pressure increases because the air molecules are traveling faster. 19.3.5. A girl uses a pump to put air into her bicycle tire at constant temperature. A pressure gauge on the pump indicates the pressure inside the tire is increasing each time she pumps. What is the cause of this pressure increase? a) The air molecules repel each other more as more molecules are added and push outward. b) The volume of the tire is constant. c) The pressure increases to keep the temperature constant. d) The pressure increasing because more air molecules are striking the walls of the tire. e) The pressure increases because the air molecules are traveling faster. 19.3.6. A closed system contains one mole of an ideal gas. Which one of the following statements is necessarily true if heat is added to the system? a) The gas must do work. b) The gas must expand, so the volume will increase, if it can. c) The gas must change phase, either from gas to liquid or from gas to solid. d) The temperature of the gas must increase. e) The conditions of the gas when the heat is added will determine the type of change that occurs. 19.3.6. A closed system contains one mole of an ideal gas. Which one of the following statements is necessarily true if heat is added to the system? a) The gas must do work. b) The gas must expand, so the volume will increase, if it can. c) The gas must change phase, either from gas to liquid or from gas to solid. d) The temperature of the gas must increase. e) The conditions of the gas when the heat is added will determine the type of change that occurs. 19.3.7. Complete the following statement: In a constant volume process, the work done is a) equal to zero joules. b) proportional to the pressure. c) proportional to the temperature. d) proportional to the volume. e) proportional to the energy transferred. 19.3.7. Complete the following statement: In a constant volume process, the work done is a) equal to zero joules. b) proportional to the pressure. c) proportional to the temperature. d) proportional to the volume. e) proportional to the energy transferred. 19.3.8. Complete the following statement: The area enclosed on a PV diagram for a given system is a) a constant value. b) equal to the heat flow into or out of the system. c) equal to the temperature change of the system. d) always equal to zero joules. e) equal to the amount of work done on or by the system. 19.3.8. Complete the following statement: The area enclosed on a PV diagram for a given system is a) a constant value. b) equal to the heat flow into or out of the system. c) equal to the temperature change of the system. d) always equal to zero joules. e) equal to the amount of work done on or by the system. 19.3.9. Consider the following conditions: (1) low temperature, (2) low density, (3) temperature near the freezing temperature, (4) temperature above the condensation temperature, and (5) high density. Under which of these conditions does a real gas behave as an ideal gas? a) 1 and 2 only b) 1 and 5 only c) 2 and 3 only d) 2 and 4 only e) A real gas can never behave as an ideal gas. 19.3.9. Consider the following conditions: (1) low temperature, (2) low density, (3) temperature near the freezing temperature, (4) temperature above the condensation temperature, and (5) high density. Under which of these conditions does a real gas behave as an ideal gas? a) 1 and 2 only b) 1 and 5 only c) 2 and 3 only d) 2 and 4 only e) A real gas can never behave as an ideal gas. 19.3.10. Why does the temperature of an ideal gas increase when the volume of the gas decreases? a) Compressing the volume does work on the gas, so its temperature must increase. b) Compressing the volume forces heat into the gas, so its temperature must increase. c) The temperature cannot change under this circumstance. d) The pressure must increase to compensate for the decreased volume. e) Since the number of moles contained within the system is constant, there will be a greater number of collisions as the volume decreases. 19.3.10. Why does the temperature of an ideal gas increase when the volume of the gas decreases? a) Compressing the volume does work on the gas, so its temperature must increase. b) Compressing the volume forces heat into the gas, so its temperature must increase. c) The temperature cannot change under this circumstance. d) The pressure must increase to compensate for the decreased volume. e) Since the number of moles contained within the system is constant, there will be a greater number of collisions as the volume decreases. 19.4.1. Which one of the following factors is directly responsible for the pressure exerted by a confined gas? a) collisions of gas molecules with the sides of the containing vessel b) atomic mass of the gas c) density of the gas d) temperature of the gas e) average translational kinetic energy of the molecules 19.4.1. Which one of the following factors is directly responsible for the pressure exerted by a confined gas? a) collisions of gas molecules with the sides of the containing vessel b) atomic mass of the gas c) density of the gas d) temperature of the gas e) average translational kinetic energy of the molecules 19.4.2. What is the meaning of the acronym rms? a) the gas constant R, the mass m, and the speed s b) root-mean-square c) rigid-massless-system d) the names of the discovers of the law: Richards, Maxwell, and Simpson e) It is derived from the Latin terms for constant pressure and volume. 19.4.2. What is the meaning of the acronym rms? a) the gas constant R, the mass m, and the speed s b) root-mean-square c) rigid-massless-system d) the names of the discovers of the law: Richards, Maxwell, and Simpson e) It is derived from the Latin terms for constant pressure and volume. 19.5.1. The absolute temperature of an ideal gas is directly proportional to which one of the following quantities? a) the number of molecules in the sample b) the average translational kinetic energy of the gas c) the relative increase in volume of the gas for a temperature increase of 1 C° d) the amount of heat required to raise the temperature of the gas by 1 C° e) the average momentum of a molecule of the gas 19.5.1. The absolute temperature of an ideal gas is directly proportional to which one of the following quantities? a) the number of molecules in the sample b) the average translational kinetic energy of the gas c) the relative increase in volume of the gas for a temperature increase of 1 C° d) the amount of heat required to raise the temperature of the gas by 1 C° e) the average momentum of a molecule of the gas 19.5.2. Which one of the following statements concerning a collection of gas molecules at a certain temperature is true? a) All molecules possess the same momentum. b) All molecules move with the same velocity. c) If the temperature is increased, the average molecular speed decreases. d) The molecules have a range of kinetic energies. e) Most of the molecules have the same kinetic energy. 19.5.2. Which one of the following statements concerning a collection of gas molecules at a certain temperature is true? a) All molecules possess the same momentum. b) All molecules move with the same velocity. c) If the temperature is increased, the average molecular speed decreases. d) The molecules have a range of kinetic energies. e) Most of the molecules have the same kinetic energy. 19.6.1. Which of the following statements provides the best definition for the term mean free path? a) The mean free path is the average distance between collisions. b) The mean free path is the average distance a molecule travels within a given time interval. c) The mean free path is the path a molecule follows within a gas. d) The mean free path is the trajectory a molecule follows after a collision. e) The mean free path is the average molecular speed within an ideal gas. 19.6.1. Which of the following statements provides the best definition for the term mean free path? a) The mean free path is the average distance between collisions. b) The mean free path is the average distance a molecule travels within a given time interval. c) The mean free path is the path a molecule follows within a gas. d) The mean free path is the trajectory a molecule follows after a collision. e) The mean free path is the average molecular speed within an ideal gas. 19.7.1. A container is filled with a large number of gas molecules at a constant temperature. The distribution (or range) of the speeds of those molecules was determined by which one of the following scientists? a) Charles b) Boyle c) Avogadro d) Maxwell e) Einstein 19.7.1. A container is filled with a large number of gas molecules at a constant temperature. The distribution (or range) of the speeds of those molecules was determined by which one of the following scientists? a) Charles b) Boyle c) Avogadro d) Maxwell e) Einstein 19.8.1. Complete the following statement: The internal energy of an ideal monatomic gas is a) dependent on both the pressure and the temperature of the gas. b) independent of the number of moles of the gas. c) proportional to the Kelvin temperature of the gas. d) a constant that is independent of pressure, volume or temperature. e) proportional to the pressure and inversely proportional to the volume of the gas. 19.8.1. Complete the following statement: The internal energy of an ideal monatomic gas is a) dependent on both the pressure and the temperature of the gas. b) independent of the number of moles of the gas. c) proportional to the Kelvin temperature of the gas. d) a constant that is independent of pressure, volume or temperature. e) proportional to the pressure and inversely proportional to the volume of the gas. 19.8.2. An ideal gas slowly expands isothermally. Which one of the following statements concerning this situation is true? a) The internal energy of the gas is equal to the amount of heat absorbed by the system. b) The work done by the gas is equal to the amount of heat absorbed by the system. c) The work done on the gas is equal to the amount of heat absorbed by the system. d) The internal energy of the gas increases by the amount of heat absorbed by the system. e) The work done on the gas is equal to the increase in the internal energy of the system. 19.8.2. An ideal gas slowly expands isothermally. Which one of the following statements concerning this situation is true? a) The internal energy of the gas is equal to the amount of heat absorbed by the system. b) The work done by the gas is equal to the amount of heat absorbed by the system. c) The work done on the gas is equal to the amount of heat absorbed by the system. d) The internal energy of the gas increases by the amount of heat absorbed by the system. e) The work done on the gas is equal to the increase in the internal energy of the system. 19.8.3. The specific heat capacity at constant volume of an ideal gas depends on which of the following parameters? a) volume b) temperature c) pressure d) number of moles of gas e) mass of the molecules 19.8.3. The specific heat capacity at constant volume of an ideal gas depends on which of the following parameters? a) volume b) temperature c) pressure d) number of moles of gas e) mass of the molecules 19.8.4. Which one of the following statements is true concerning the ratio of the molar heat capacities CP/CV for an ideal gas? a) The ratio is sometimes less than or equal to 1. b) The ratio is sometimes greater than 1. c) The ratio is always equal to 1. d) The ratio is always less that 1. e) The ratio is always greater than 1. 19.8.4. Which one of the following statements is true concerning the ratio of the molar heat capacities CP/CV for an ideal gas? a) The ratio is sometimes less than or equal to 1. b) The ratio is sometimes greater than 1. c) The ratio is always equal to 1. d) The ratio is always less that 1. e) The ratio is always greater than 1. 19.8.5. Which of the following choices gives the molar specific heat at constant volume CV for an ideal monatomic gas in terms of the universal gas constant R? a) 5R/3 b) 5R/2 c) 3R/5 d) 3R/2 e) 7R/3 19.8.5. Which of the following choices gives the molar specific heat at constant volume CV for an ideal monatomic gas in terms of the universal gas constant R? a) 5R/3 b) 5R/2 c) 3R/5 d) 3R/2 e) 7R/3 19.8.6. Which of the following choices gives the molar specific heat at constant pressure CP for an ideal monatomic gas in terms of the universal gas constant R? a) 5R/3 b) 5R/2 c) 3R/5 d) 3R/2 e) 7R/3 19.8.6. Which of the following choices gives the molar specific heat at constant pressure CP for an ideal monatomic gas in terms of the universal gas constant R? a) 5R/3 b) 5R/2 c) 3R/5 d) 3R/2 e) 7R/3 19.8.7. On which of the following parameters does the heat contained within an ideal gas depend? a) temperature b) mean free path c) pressure d) volume e) Gases do not have heat. 19.8.7. On which of the following parameters does the heat contained within an ideal gas depend? a) temperature b) mean free path c) pressure d) volume e) Gases do not have heat. 19.8.8. On which of the following parameters does the internal energy within an ideal gas depend? a) temperature b) mean free path c) pressure d) volume e) Gases do not have heat. 19.8.8. On which of the following parameters does the internal energy within an ideal gas depend? a) temperature b) mean free path c) pressure d) volume e) Gases do not have heat. 19.9.1. Complete the following statement: On average, a molecule can store an energy of per molecule for each a) atom in the molecule. b) degree of freedom. c) collision that occurs per unit time. d) mole contained within the system. e) free electron in the system. 19.9.1. Complete the following statement: On average, a molecule can store an energy of per molecule for each a) atom in the molecule. b) degree of freedom. c) collision that occurs per unit time. d) mole contained within the system. e) free electron in the system. 19.11.1. An ideal gas slowly expands adiabatically. Which one of the following statements concerning this situation is true? a) Work is done on the gas. b) The temperature of the system remains constant. c) The pressure of the system remains constant. d) Heat is neither added nor removed from the system. e) The density of the gas remains constant. 19.11.1. An ideal gas slowly expands adiabatically. Which one of the following statements concerning this situation is true? a) Work is done on the gas. b) The temperature of the system remains constant. c) The pressure of the system remains constant. d) Heat is neither added nor removed from the system. e) The density of the gas remains constant. 19.11.2. Which of the following processes requires the largest amount of work to be done on a system containing an ideal gas if the volume of the system is reduced to one-half of its initial value? a) constant pressure b) isothermal c) adiabatic d) isothermal and constant pressure require the same amount of work e) isothermal and adiabatic require the same amount of work 19.11.2. Which of the following processes requires the largest amount of work to be done on a system containing an ideal gas if the volume of the system is reduced to one-half of its initial value? a) constant pressure b) isothermal c) adiabatic d) isothermal and constant pressure require the same amount of work e) isothermal and adiabatic require the same amount of work 19.11.3. Complete the following statement: During an adiabatic process involving an ideal gas, a) no work is done by the gas. b) no heat flow occurs. c) no volume change occurs. d) no pressure change occurs. e) no temperature change occurs. 19.11.3. Complete the following statement: During an adiabatic process involving an ideal gas, a) no work is done by the gas. b) no heat flow occurs. c) no volume change occurs. d) no pressure change occurs. e) no temperature change occurs. 19.11.4. An isochoric process occurs when which of the following parameters is constant during the process? a) volume b) pressure c) temperature d) work e) heat 19.11.4. An isochoric process occurs when which of the following parameters is constant during the process? a) volume b) pressure c) temperature d) work e) heat 19.11.5. An isobaric process occurs when which of the following parameters is constant during the process? a) volume b) pressure c) temperature d) work e) heat 19.11.5. An isobaric process occurs when which of the following parameters is constant during the process? a) volume b) pressure c) temperature d) work e) heat