File
advertisement

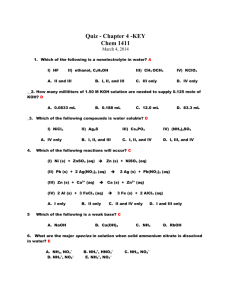
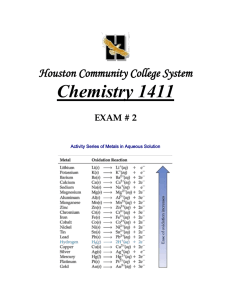
Indicator Colour of Colour of pH Range Acid Form Base Form Phenolpht Colourless Pink halein 8.2-10.0 Methyl Orange Pink Yellow 3.2-4.4 Bromothy mol Blue Yellow Blue 6.0-7.6 -The indicator must be chosen carefully to ensure that the point during the titration where the indicator changes colour, the end point, closely matches the equivalence point of the reaction. - If we look at the pH changes that take place between a strong acid and a strong base, we would see a graph similar to 4.4a (page 40). At the end point, addition of a very small volume of strong acid produces a large change in pH. This is referred to as the sharp end point. -In the case between a strong acid and base, the equivalence point falls close to 7.0 A 1.50g sample of lawn fertiliser was boiled with 25.00mL of 0.9987M sodium hydroxide solution. When no further ammonia gas was evolved from the mixture, it as cooled and titrated with 0.2132M hydrochloric acid, using phenolphthalein as an indicator. A titre of 19.78mL was required. Calculate the percentage of ammonium ions in the fertiliser. STEP 1: Find the original amount of NaOH used. STEP 2: Find the amount of NaOH that did not react with NH4+ ions. STEP 3: Find the amount of NaOH that reacted with NH4+ ions. STEP 4: Find the amount of NH4+ in the fertiliser sample. STEP 5: Find the percentage of NH4+ in the sample.