Janeway's Immunology - Cal State LA
advertisement

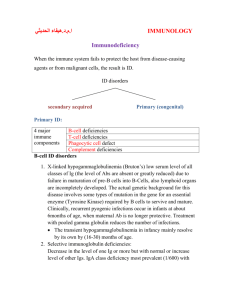
MICR 304 Immunology & Serology Lecture 16 Immunodeficiency Diseases Chapter 12.7- Overview of Today’s Lecture • Inherited immunodeficiencies – Immunoglobulins – Complement – Phagocytes – T cells • Acquired immunodeficiencies – Artificial – AIDS Immunodeficiencies • Caused by defects of one or more components of the immune system • Recurrent infections • Often opportunistic microbes Susceptibility for Infections • Defects in complement, phagocytes, antibodies: – Extracellular bacteria, pyogenic infections • Defects in T-cells: – Fungal and viral infections, infections with intracellular pathogens Compare and Contrast Inherited and Acquired Immune Deficiencies • Inherited defects of the immune system – Alterations in genes involved in immunity – Symptomatic from infancy on • Acquired defects of the immune system – Various causes – Typically develop later Our most Important Defense Weapons • Complement: to opsonize, initiate inflammatory response, and to kill • Antimicrobial peptides: to kill and to modulate immune response • Phagocytes: to remove microbes, prevent further spread, to kill and in some cases to initiate adaptive immune response • Antibodies: to neutralize, opsonize, and to agglutinate • Granuloma formation: a concerted action to wall off and ultimately kill intracellular microbes • NK cells and CTLs: to remove infected (or maligne) host cells via apoptosis and indirectly kill microbial invaders • TH and regulatory T cells: to orchestrate the immune response Evaluation of the Immune System Inherited Immune Defects Discussed Today Complement C1 Inhibitor deficiency, C5-9 deficiency Phagocytes Chronic granulomatous disease Leukocyte adhesion deficiency Antibodies (B-cells) X-linked ammaglobulinemia X-linked hyper IgM syndrome T- cells X-linked gc SCID B+T cells ADA deficiency Complement System C1INH DAF Factor I Proctectin Complement Deficiencies • Lack of active complement factors: – C3 damage: wide range of pyogenic infections (S. aureus, S. pyogenes etc) – Overall and in particular with C5-9 deficiency increased Neisseria infections • 10,000 x risk increase • Lack of control proteins – C1 Inhibitor: angioneurotic edema – Decay accelerating factor: spontaneous hemolytic attacks – Factor I: consumption of complement, lack of complement Phagocyte Defects • Chronic Granulomatous Disease – NADPH oxidase defect • Many different gene mutations – No superoxide radical production – Host forms granulomas to eliminate pathogens • Leukocyte adhesion deficiency – Mutations in sialyl lewis (ligand for selectin) or intergrins – Prevents leukocyte migration to the locus of infection Diagnostics in Chronic Granulomatous Disease Altered NBT Test Pneumonia Normal Patient Carrier Nitro blue tetrazolium is reduced by NADPH oxidase to yield an insoluble blue formazan salt. Aspergillus pneumonia in CGD patient X-Linked Agammaglobulinemia (XLA) • Failure to produce antibodies – Increase in infections with pyogenic bacteria e.g. S. pyogenes and chronic viral infections e.g. hepatitis B • • • • Mutations in X-chromosome One of the common: Bruton’s Disease Mutation in Bruton’s tyrosine kinase (Btk) Signal transduction defect in pre-B cells – No stimulation in response to Ag – Arrest of B-cell development – B-cells rarely found in peripheral blood Consequences of Btk Mutation • In normal males, only Xchromosome is active • In affected male no B-cell development • In female carriers, only Bcells that have randomly inactivated the defect chromosome mature – All mature B-cells have the nondefective x-chromosome activated – Non-random X chromosome inactivation only in B cells Signal transduced via Btk Onset of XLA • When maternal antibodies fade Absence of Immunoglobulins in Serum from XLA Patients Absence of B-Cells in XLA • Flow cytometer analysis • CD19: B-cell marker • CD3: T-cell marker Refresher: B-Cell Activation by T-Cells Hyper IgM Syndrome Selected syndromes • Normal B and T cell development but lack of IgG, IgA, and IgE • B-cell activation by T-cells is disrupted – CD40 ligand deficiency in T-cells prevents activation of otherwise normal B-cells • X-linked • Also defective in cell mediated immunity – Mutations in CD40 in B cells – Mutation of NFkB pathways in B-cells (NEMO) – AID deficiency • No isotype switch after antigen recognition • Lymphoid tissues are devoid of germinal centers AID Deficiency • Mutation in gene for activation induced cytidine deaminase • Subform of hyper IgM syndrome • Defective B cells with lack of isotype switch • More susceptible to severe bacterial infections but not to opportunistic infections e.g. P. carinii pneumonia Severe Combined Immunodeficiencies • B and T cell function is affected • Causes can be primary T cell defects or T and B cell defects • Without T cells lack of – T cell dependent antibodies – Cell mediated immune responses – Immunological memory • Severe opportunistic infections – – – – Adenoviruses EBV Candida albicans P. carinii Major Causes of SCID X-Linked SCID with Common Gamma Chain Mutation • Common gamma chain of the following cytokine receptors affected: – IL2, IL4, IL7, IL9, IL15, IL21 • Predominantly T-cell defect • No T-cell development, no NK cells • B cell numbers are normal but not their function • Lack of macrophage activation and B-cell activation • X-linked Refresher: Selected Cytokines Cytokine Major Effects IL2 T cell proliferation IL4 B cell activation, IgE switch, TH2 differentiation IL7 Growth of pre-B cells and pre-T cells IL9 Mast cell enhancing activity, TH2 stimulation IL15 Stimulates growth of T cells, NK cells, enhances CD8 memory T cell survival IL21 Induces proliferation of B, T, and NK cells Autosomal SCID: ADA Deficiency • Adenosine deaminase deficiency • Alterations in purine degradation • Accumulation of nucleotide metabolites – In particular toxic for T-cells, also B-cells • General lack of T and B-cell function Refresher: T-Cells and Granuloma Formation Functional T cells are essential in clearing infections with intracellular pathogens. Acquired Immune Deficiencies • Natural: newborns • Immune suppressive therapy – Cyclosporin (transplantation) – Steroids (autoimmune diseases, chronic inflammation) • Tumor patients – Neutropenia during therapy – Cytokine production (TGF-b, IL10) • Chronic Disease • Infections – Measles – HIV and AIDS Transient Immunoglobulin Deficiency in Newborns HIV Infection is Pandemic HIV Virus • Retrovirus • Infects CD4+ cells: TH cells, dendritic cells, macrophages, monocytes • Requires co-receptor: chemokine receptor – Tropism depends on chemokine receptor – CCR5: DC, MP, CD4 T Ly • R5 virus • Mutations protect against HIV infection – CXCR4: activated T Ly • X4 virus Dendritic Cells Transport HIV to Lymphnodes Typical Course of Untreated HIV Infection Immune Response to HIV HIV Associated Diseases •T- cell defect •Lack of macrophage activation •Intracellular opportunistic infections •Lack of effective CTLs •Development of virusassociated tumors Tumors Associated with HIV Infection • Remember – T-helper cells activate NK cells • New Herpes viridae are involved that are not eliminated • Lymphoma, Kaposi sarkoma Diagnostics of Immune Deficiencies • Complement: CH50 test • Phagocytes: NBT test, phagocytosis and killing tests • Antibodies: immune electrophoresis • B-cells: pokeweed stimulation, induced antibody response • T cells: unspecific lymphocyte stimulation with phytohemagglutin, skin tests Therapeutic Approaches for Immune Deficiencies • • • • Symptomatic Ig treatment Bone marrow transplantation Gene therapy – Remove bone marrow – Insert new gene into collected cells – Re-implant bone marrow • Antiviral therapy Bone Marrow Grafting is Problematic Can be prevented by T-cell depletion of donor marrow HIV Therapy • Reverse Transcriptase Inhibitors • Protease Inhibitors • Virus decoys (material to which virus binds instead of to cells) • Boosting immune system Often requires up to 40 pills a day.