Redox Reactions & Electrochemistry: Lecture Notes
advertisement
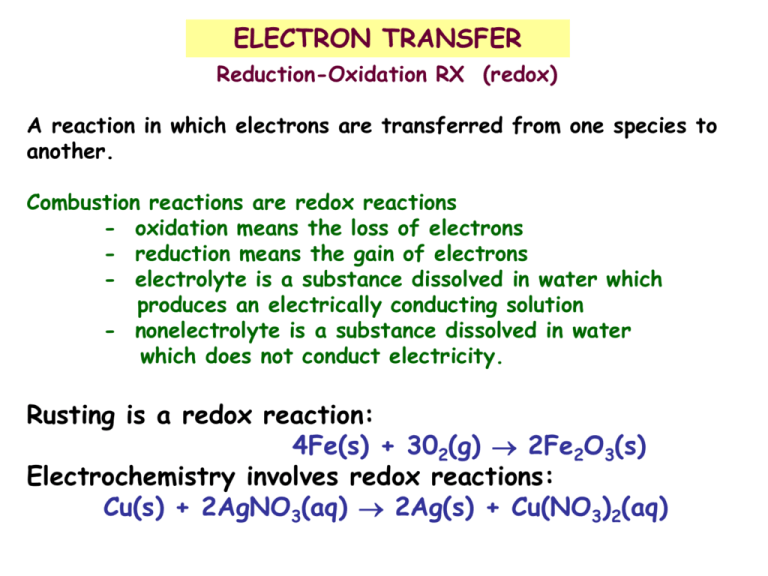
ELECTRON TRANSFER Reduction-Oxidation RX (redox) A reaction in which electrons are transferred from one species to another. Combustion reactions are redox reactions - oxidation means the loss of electrons - reduction means the gain of electrons - electrolyte is a substance dissolved in water which produces an electrically conducting solution - nonelectrolyte is a substance dissolved in water which does not conduct electricity. Rusting is a redox reaction: 4Fe(s) + 302(g) 2Fe2O3(s) Electrochemistry involves redox reactions: Cu(s) + 2AgNO3(aq) 2Ag(s) + Cu(NO3)2(aq) REDOX Rx (reduction-oxidation reactions) “transfer of electrons” Oxidation = Loss of electrons Reduction = Gain of electrons 2Mg + O2 2MgO OXIDATION STATES 1. All elements have an oxidation number of zero. 2. Group I and II have +1, +2 respectively 3. Oxygen has -2, Flouride has -1 0 3+ -2 0 3+ 2- 2Al + Fe2O3 2Fe + Al2O3 IDENTIFING REDOX RX Element + compound New element + New compound A + BC B + AC Element + Element Compound A + B AB Check oxidation state (charges) of species A change in oxidation # means redox reaction Identify the Redox Rx: Cu + AgNO3 Cu(NO3)2 + Ag NO + O2 NO2 K2SO4 + CaCl2 KCl + CaSO4 C2H4O2 + O2 CO2 + H2O LABELING COMPONENTS OF REDOX REACTIONS The REDUCING AGENT is the species which undergoes OXIDATION. The OXIDIZING AGENT is the species which undergoes REDUCTION. CuO + H2 Cu + H2O A summary of redox terminology. OXIDATION One reactant loses electrons. Zn loses electrons. Reducing agent is oxidized. Zn is the reducing agent and becomes oxidized. Oxidation number increases. The oxidation number of Zn increases from x to +2. REDUCTION Other reactant gains electrons. Oxidizing agent is reduced. Hydrogen ion gains electrons. Hydrogen ion is the oxidizing agent and becomes reduced. Oxidation number decreases. The oxidation number of H decreases from +1 to 0. Key Points About Redox Reactions •Oxidation (electron loss) always accompanies reduction (electron gain). •The oxidizing agent is reduced, and the reducing agent is oxidized. •The number of electrons gained by the oxidizing agent always equals the number lost by the reducing agent. REDOX REACTIONS For the following reactions, identify the oxidizing and reducing agents. MnO4- + C2O42- MnO2 + CO2 acid: Cr2O72- + Fe2+ Cr3+ + Fe3+ base: CO2+ + H2O2 CO(OH)3 + H2O As + ClO3- H3AsO3 + HClO Strongest oxidizing agent Oxidizing/Reducing Agents Most positive values of E° red F2(g) + 2e- • • H2(g) • + Li(s) Most negative values of E° red Li+(aq) Increasing strength of reducing agent • 2H+(aq) + 2e- Increasing strength of oxidizing agent 2F-(aq) e- Strongest reducing agent More Positive E º Cathode(reduction) Red Eº Red (cathode) (V) Eº cell Eºred (anode) Anode(oxidation) More Negative Standard Reduction Potentials in Water at 25°C Standard Potential (V) 2.87 1.51 1.36 1.33 1.23 1.06 0.96 0.80 0.77 0.68 0.59 0.54 0.40 0.34 0 -0.28 -0.44 -0.76 -0.83 -1.66 -2.71 -3.05 Reduction Half Reaction F2(g) + 2e- 2F-(aq) MnO4-(aq) + 8H+(aq) + 5e- Mn2+(aq) + 4H2O(l) Cl2(g) + 2e- 2Cl-(aq) Cr2O72-(aq) + 14H+(aq) + 6e- 2Cr3+(aq) + 7H2O(l) O2(g) + 4H+(aq) + 4e- 2H2O(l) Br2(l) + 2e- 2Br-(aq) NO3-(aq) + 4H+(aq) + 3e- NO(g) + H2O(l) Ag+(aq) + e- Ag(s) Fe3+(aq) + e- Fe2+(aq) O2(g) + 2H+(aq) + 2e- H2O2(aq) MnO4-(aq) + 2H2O(l) + 3e- MnO2(s) + 4OH-(aq) I2(s) + 2e- 2I-(aq) O2(g) + 2H2O(l) + 4e- 4OH-(aq) Cu2+(aq) + 2e- Cu(s) 2H+(aq) + 2e- H2(g) Ni2+(aq) + 2e- Ni(s) Fe2+(aq) + 2e- Fe(s) An2+(aq) + 2e- Zn(s) 2H2O(l) + 2e- H2(g) + 2OH-(aq) Al3+(aq) + 3e- Al(s) Na+(aq) + e- Na(s) Li+(aq) + e- Li(s) ELECTROCHEMISTRY A system consisting of electrodes that dip into an electrolyte and in which a chemical reaction uses or generates an electric current. Two Basic Types of Electrochemical cells: Galvanic (Voltaic) Cell: A spontaneous reaction generates an electric current. Chemical energy is converted into electrical energy Electrolytic Cell: An electric current drives a nonspontaneous reaction. Electrical energy is converted into chemical energy. ELECTROCHEMICAL CELLS CHEMICALS AND EQUIPMENT NEEDED TO BUILD A SIMPLE CELL: The Cell: Voltmeter Two beakers or glass jars Two alligator clips The Electrodes: Metal electrode Metal salt solution The Salt Bridge: Glass or Plastic u-tube Na or K salt solution ELECTROCHEMICAL CELLS A CHEMICAL CHANGE PRODUCES ELECTRICITY Theory: If a metal strip is placed in a solution of it’s metal ions, one of the following reactions may occur Mn+ + ne- M M Mn+ + neThese reactions are called half-reactions or half cell reactions If different metal electrodes in their respective solutions were connected by a wire, and if the solutions were electrically connected by a porous membrane or a bridge that minimizes mixing of the solutions, a flow of electrons will move from one electrode, where the reaction is M1 M1n+ + neTo the other electrode, where the reaction is M2n+ + ne- M2 The overall reaction would be M1 + M2n+ M2 + M1n+ General characteristics of voltaic and electrolytic cells. VOLTAIC CELL System Energydoes is released work on from its spontaneous surroundings redox reaction Oxidation half-reaction X X+ + e- ELECTROLYTIC CELL Surroundings(power Energy is absorbed tosupply) drive a nonspontaneous redox reaction do work on system(cell) Oxidation half-reaction AA + e- Reduction half-reaction Y++ e- Y Reduction half-reaction B++ eB Overall (cell) reaction X + Y+ X+ + Y; DG < 0 Overall (cell) reaction A- + B+ A + B; DG > 0 The electrolysis of water: nonspontaneous Overall (cell) reaction 2H2O(l) H2(g) + O2(g) Oxidation half-reaction 2H2O(l) 4H+(aq) + O2(g) + 4e- Reduction half-reaction 2H2O(l) + 4e2H2(g) + 2OH-(aq) The spontaneous reaction between zinc and copper(II) ion. ACTIVITY SERIES OF SOME SELECTED METALS A brief activity series of selected metals, hydrogen and halogens are shown below. The series are listed in descending order of chemical reactivity, with the most active metals and halogens at the top (the elements most likely to undergo oxidation). Any metal on the list will replace the ions of those metals (to undergo reduction) that appear anywhere underneath it on the list. METALS HALOGENS K (most oxidized Ca Na Mg Al Zn Fe Ni Sn Pb H Cu Ag Hg Au(least oxidized) F2 Cl2 Br2 l2 Oxidation refers to the loss of electrons and reduction refers to the gain of electrons The reaction of calcium in water: spontaneous Oxidation half-reaction Ca(s) Ca2+(aq) + 2e- Reduction half-reaction 2H2O(l) + 2eH2(g) + 2OH-(aq) Overall (cell) reaction Ca(s) + 2H2O(l) Ca2+(aq) + H2(g) + 2OH-(aq) Activity Series Relative Reactivities (Activities) of Metals 1. Metals that can displace H from acid 2. Metals that cannot displace H from acid 3. Metals that can displace H from water 4. Metals that can displace other metals from solution K Ba Ca Na Mg Al Zn Cr Fe Ni Sn Pb H2 Cu Ag Hg Au A voltaic cell based on the zinc-copper reaction. Oxidation half-reaction Zn(s) Zn2+(aq) + 2e- Reduction half-reaction Cu2+(aq) + 2eCu(s) Overall (cell) reaction Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) Why Does a Voltaic Cell Work? The spontaneous reaction occurs as a result of the different abilities of materials (such as metals) to give up their electrons and the ability of the electrons to flow through the circuit. Ecell > 0 for a spontaneous reaction 1 Volt (V) = 1 Joule (J)/ Coulomb (C) Notation for a Voltaic Cell components of anode compartment components of cathode compartment (oxidation half-cell) (reduction half-cell) phase of lower phase of higher oxidation state oxidation state phase of higher oxidation state phase of lower oxidation state phase boundary between half-cells Zn(s) | Zn2+(aq) || Cu2+(aq) | Cu (s) Examples: Zn(s) Zn2+(aq) + 2e- Cu2+(aq) + 2e- Cu(s) graphite | I-(aq) | I2(s) || H+(aq), MnO4-(aq) | Mn2+(aq) | graphite inert electrode NOTATION FOR VOLTAIC CELLS Zn + Cu2+ Zn2+ + Cu Zn(s)/Zn2+(aq) // Cu2+(aq)/Cu(s) Anode Cathode oxidation reduction salt bridge write the net ionic equation for: Al(s)/Al3+(aq)//Cu2+(aq)/Cu(s) Sample Problem: PROBLEM: PLAN: Diagramming Voltaic Cells Diagram, show balanced equations, and write the notation for a voltaic cell that consists of one half-cell with a Cr bar in a Cr(NO3)3 solution, another half-cell with an Ag bar in an AgNO3 solution, and a KNO3 salt bridge. Measurement indicates that the Cr electrode is negative relative to the Ag electrode. Identify the oxidation and reduction reactions and write each halfreaction. Associate the (-)(Cr) pole with the anode (oxidation) and the (+) pole with the cathode (reduction). Voltmeter e- SOLUTION: Oxidation half-reaction Cr(s) Cr3+(aq) + 3e- salt bridge Cr Ag K+ NO3- Reduction half-reaction Ag+(aq) + eAg(s) Cr3+ Ag + Overall (cell) reaction Cr(s) + Ag+(aq) Cr3+(aq) + Ag(s) Cr(s) | Cr3+(aq) || Ag+(aq) | Ag(s) Voltages of Some Voltaic Cells Voltaic Cell Voltage (V) Common alkaline battery 1.5 Lead-acid car battery (6 cells = 12V) 2.0 Calculator battery (mercury) 1.3 Electric eel (~5000 cells in 6-ft eel = 750V) 0.15 Nerve of giant squid (across cell membrane) 0.070 Alkaline Battery Mercury and Silver (Button) Batteries Lithium battery. Nickel-metal hydride (Ni-MH) battery Lithium-ion battery. A voltaic cell using inactive electrodes. Oxidation half-reaction 2I-(aq) I2(s) + 2e- Reduction half-reaction MnO4-(aq) + 8H+(aq) + 5eMn2+(aq) + 4H2O(l) Overall (cell) reaction 2MnO4-(aq) + 16H+(aq) + 10I-(aq) 2Mn2+(aq) + 5I2(s) + 8H2O(l) The Hydrogen Electrode: At the hydrogen electrode, the half reaction involves a gas. 2 H+(aq) + 2e- H2(g) so an inert material must serve as the reaction site (Pt) H+(aq)/H2(g)/Pt cathode Pt/H2(g)/H+(aq) anode Therefore: Al(g)/Al3+(aq)//H+(aq)/H2(g)/Pt Determining an unknown E0half-cell with the standard reference (hydrogen) electrode. Oxidation half-reaction Zn(s) Zn2+(aq) + 2e- Overall (cell) reaction Zn(s) + 2H3O+(aq) Zn2+(aq) + H2(g) + 2H2O(l) Reduction half-reaction 2H3O+(aq) + 2eH2(g) + 2H2O(l) The laboratory measurement of pH. Pt Glass electrode Reference (calomel) electrode Hg Paste of Hg2Cl2 in Hg AgCl on Ag on Pt 1M HCl Thin glass membrane KCl solution Porous ceramic plugs Rechargeable Batteries The tin-copper reaction as the basis of a voltaic and an electrolytic cell. voltaic cell Oxidation half-reaction Sn(s) Sn2+(aq) + 2eReduction half-reaction Cu2+(aq) + 2eCu(s) Overall (cell) reaction Sn(s) + Cu2+(aq) Sn2+(aq) + Cu(s) electrolytic cell Oxidation half-reaction Cu(s) Cu2+(aq) + 2eReduction half-reaction Sn2+(aq) + 2eSn(s) Overall (cell) reaction Sn(s) + Cu2+(aq) Sn2+(aq) + Cu(s) Lead-acid battery. The processes occurring during the discharge and recharge of a lead-acid battery. VOLTAIC(discharge) ELECTROLYTIC(recharge) The corrosion of iron. OTHER APPLICATIONS FOR REDOX REACTIONS Corrosion Photosynthesis Cleaning agents and other household supplies Chemicals The effect of metal-metal contact on the corrosion of iron. faster corrosion In Photosynthesis, carbohydrates are produced in the green leaves of plants carbon cycle 6CO2 + 6H2O + hv (photosynthesis) C6H12O6 + 6O2 (respiration) glucose In body tissues, Glucose is oxidized in metabolic reactions (respiration) and chemical energy is released to do work in the cells. Polysaccharides react with water to yield monosaccharides, (Hydrolysis) H+ C12H22O11 + H2O C6H12O6 + C6H12O6 sucrose glucose fructose COMMON OXIDIZING AGENTS - O2 (gaseous) H2O2 (antiseptics) tincture of Iodine K2Cr2O7 (breathalyzer) benezoyl peroxide Ca (Ocl)2 (swimming pools) disinfectant NaOCl (bleaches) COMMON REDUCING AGENTS - coal (coke) for producing metals from ores - more reactive metals (ore) - H2 (ores & hydrocarbons) - B/W photograph developer C6H4(OH)2 + 2 Ag+ C6H4O2 + 2Ag + 2 H+ - antioxidants ascorbic acid “C” tocopherol “E” beta-carotene “A” HYDROGEN H2 - most abundent element in universe - rare on earth - flammable as an element - usually found as compound Acids = HCl hydrocarbons = CH4 water = H2O - many times required a catalyst to react. Catalyst speeds up the reaction but is not consumed. (Pt, Ni, Pd) Pt CuO + H2 Cu + H2O - produced by reacting an acid with a reactive metal Mg + 2HCl MgCl2 + H2 OXYGEN O2 - occurs in atmosphere, hydrosphere , and lithosphere O2 H2O SiO2 - percent composition of H2O = 89% O 2/3 body weight is O - needed for combustion HC + O2 CO2 + H2O - used in rusting or corrosion 2Cu + O2 2CuO 4Fe + 3O2 2Fe2O3 - nonmetal oxides C + O2 CO2 S + O2 SO2 - needed for burning