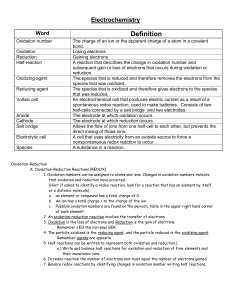
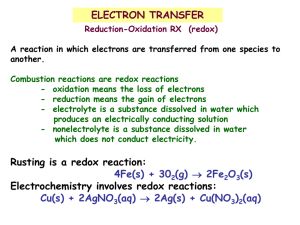
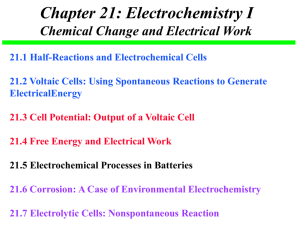
Chemistry 30 - Outcomes Unit 2
advertisement
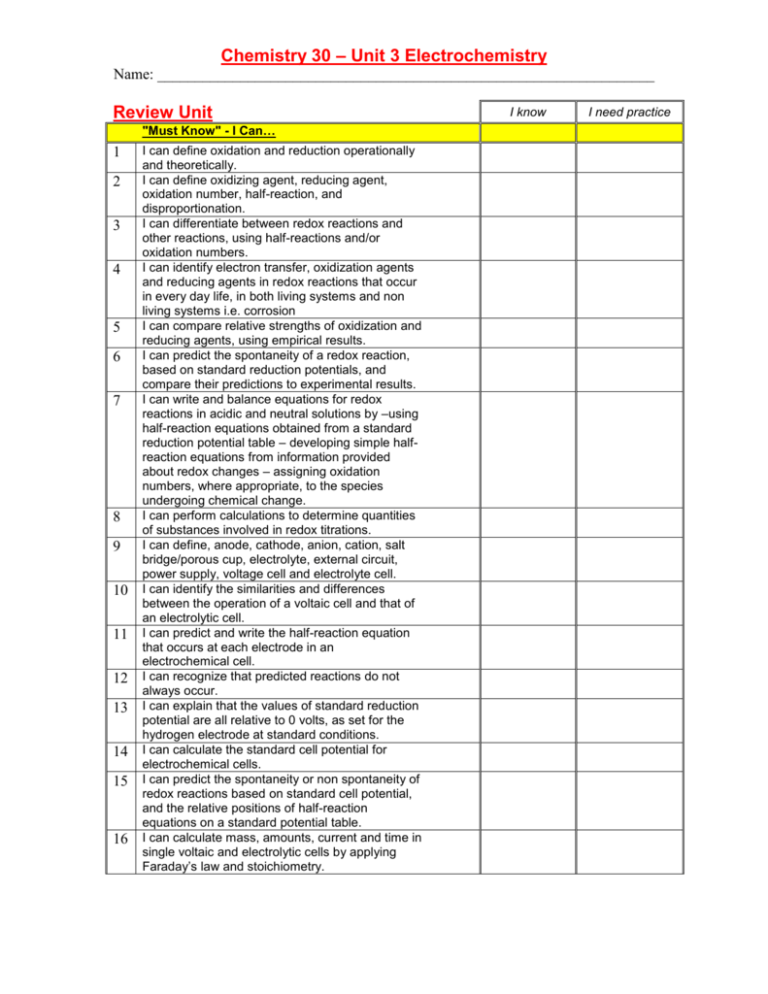
Chemistry 30 – Unit 3 Electrochemistry Name: __________________________________________________________________ Review Unit "Must Know" - I Can… 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 I can define oxidation and reduction operationally and theoretically. I can define oxidizing agent, reducing agent, oxidation number, half-reaction, and disproportionation. I can differentiate between redox reactions and other reactions, using half-reactions and/or oxidation numbers. I can identify electron transfer, oxidization agents and reducing agents in redox reactions that occur in every day life, in both living systems and non living systems i.e. corrosion I can compare relative strengths of oxidization and reducing agents, using empirical results. I can predict the spontaneity of a redox reaction, based on standard reduction potentials, and compare their predictions to experimental results. I can write and balance equations for redox reactions in acidic and neutral solutions by –using half-reaction equations obtained from a standard reduction potential table – developing simple halfreaction equations from information provided about redox changes – assigning oxidation numbers, where appropriate, to the species undergoing chemical change. I can perform calculations to determine quantities of substances involved in redox titrations. I can define, anode, cathode, anion, cation, salt bridge/porous cup, electrolyte, external circuit, power supply, voltage cell and electrolyte cell. I can identify the similarities and differences between the operation of a voltaic cell and that of an electrolytic cell. I can predict and write the half-reaction equation that occurs at each electrode in an electrochemical cell. I can recognize that predicted reactions do not always occur. I can explain that the values of standard reduction potential are all relative to 0 volts, as set for the hydrogen electrode at standard conditions. I can calculate the standard cell potential for electrochemical cells. I can predict the spontaneity or non spontaneity of redox reactions based on standard cell potential, and the relative positions of half-reaction equations on a standard potential table. I can calculate mass, amounts, current and time in single voltaic and electrolytic cells by applying Faraday’s law and stoichiometry. I know I need practice Chemistry 30 – Unit 3 Electrochemistry Name: __________________________________________________________________ Key vocabulary: 1. 2. 3. 4. 5. Oxidation Reduction Oxidation agent Reduction agent Oxidation number 6. Half-reaction 7. Disproportionati on 8. Redox reaction 9. Standard reduction potential table 10. Redox titrations 11. Anode 12. Cathode 13. Anion 14. Cation 15. Salt bridge 16. Porous cup 17. Electrolyte 18. External circuit 19. Power supply 20. Voltaic cell 21. Electrolytic cell 22. Standard reduction potential 23. Faraday’s law