2CHM - Summ Prac EASTER
advertisement

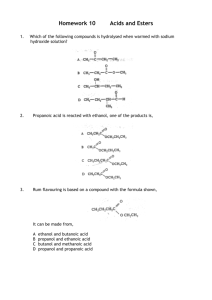
STAGE 2 CHEMISTRY PRACTICAL - ESTER PREPARATION ASSESSMENT TYPE 3: Investigations Folio Practical Investigation: Preparation of an Ester Purpose This task allows you to demonstrate your ability to prepare an organic compound. Performance Standards which this practical activity addresses: I3 AE2 A2 Manipulation of apparatus to implement safe and ethical investigation procedures Evaluation of procedures, with suggestions for improvement. use of appropriate chemistry terms, conventions, formulae, and equations. A3 Demonstrations of skills in individual and collaborative work. KU3 Communication of knowledge and understanding of chemistry in different formats Description of assessment Using the method provided on the attached sheet, this preparation will assess: • the well-organised, safe and effective manipulation of apparatus for reflux, distillation, liquid-liquid extraction procedures • the quality of the product prepared • the chemical reasoning behind the procedures followed • the reasoning behind the suggested improvements • the correct use of chemical terms, conventions, formulae, and equations • skills in individual and collaborative work • the communication skills in your report. Assessment conditions The preparation will be conducted individually DUE DATE – Tuesday 21st August 2012 Assessment Structure for ester preparation practical: Purpose: To prepare an ester and to purify it. Materials: Quickfit apparatus measuring cylinders (10 mL, 25 mL) glacial acetic acid (ethanoic acid pure) 1 - pentanol concentrated sulphuric acid boiling chips 20 mL sodium carbonate solution (0.1 M) fused calcium chloride small conical flask and stopper 200 C thermometer Note Glacial acetic acid : corrosive and flammable 1-pentanol: vapours are harmful Concentrated sulphuric acid: extremely corrosive Procedure PART- A Preparation of Ester 1. Measure 15 mL of the alcohol provided and 10 mL ethanoic acid (pure) into the 50 mL pear-shaped Quickfit flask. 2. Slowly add about 1 mL concentrated sulphuric acid and a few boiling chips. 3. Assemble the glassware for reflux. 4. Reflux the mixture for 30 minutes, adjusting the flame to ensure smooth boiling. 5. Allow flask and contents to cool. Part B: Isolation of Pure Ester 1. Pour the cooled mixture into a separating funnel, which contains about 20 mL of water. Shake and allow the layers to separate. Check which is the aqueous layer. 2. Collect the organic layer and wash it second time with water. 3. Collect the organic layer and wash it with about 20 mL of sodium carbonate solution. 4. Repeat this step until no more effervescence occurs. Take care with this step as pressure is built up due to carbon dioxide release. Take care with this step. 5. Collect the organic layer and wash it with about 20 mL of distilled water. 6. Collect the organic layer in a small conical flask containing fused calcium chloride (a dehydrating agent). Stopper, swirl and allow to stand for 15 minutes. 7. Decant the organic liquid into a clean, dry 50 mL pear-shaped flask and add boiling chips. 8. Assemble the glassware for distillation. 9. Distil the ester, recording the boiling range of the fractions collected. Do not distil all of the liquid. The boiling points of the possible components of the mixture to be distilled are: Boiling Point (C) 10 ethanoic acid 118 1-pentanol 137 pure ester 149 sulphuric acid 142 Record the appearance and odour of the ester in the fraction most likely to contain it. Part C: PRACTICAL REPORT When you have completed your practical you are to write a practical report using the two headings, ‘Results’, and ‘Discussion’. A guide to what is to be included under each of these headings is indicated below. When you submit your report attach to it your laboratory notes and the feedback sheet. Results: Record the boiling range of your distillate and relevant observations. Present your product for the teacher to inspect. Discussion: In your discussion you should: 1. Write an equation for the esterification reaction and state the systematic name of the ester formed with its structural formula. 2. Explain the purpose of: (a) the reflux (b) all isolation procedures, using equations where appropriate 3. Explain why this method of preparation usually results in a poor yield of ester 4. Suggest modifications that would : (a) improve the yield of ester (b) improve the purity of the ester 5. Identify two hazards associated with this preparation and describe the precautions taken to ensure safety. 6. Briefly review the effectiveness with which you and your partner worked as a team, any suggestions for improvements in your team work. STAGE 2 CHEMISTRY PRACTICAL– ESTER PREPARATION Performance standard I3 Assessment Criteria Grade Practical Skills Checklists (Teacher observations) Performing reflux Use of separating funnel Performing distillation A,B,C,D,E,N Safe working procedure Instructions followed accurately Area cleanliness Cooperation Clarity of ester product Boiling range of distillate & relevant observations like physical characteristics and yield (by students) A2 A,B,C,D,E,N Correct structural formula of Ester Ester named correctly Correct equations written for Esterification Reaction Other reactions A3 Individual & Collaborative work skills AE2 Discussion: Explain the purpose of: reflux all isolation procedures. A,B,C,D,E,N A,B,C,D,E,N Explanation given for poor yield. Suggestions given for modifications Hazards identified and appropriate precautions stated. KU3 A,B,C,D,E,N Communication skills and understanding of A,B,C,D,E,N chemistry in different formats OVERALL GRADE Teacher’s comment