Isomerism - Rupali Handal | The Pharmacist..
advertisement

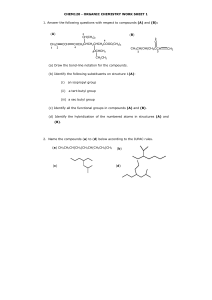
ISOMERISM 1 Contents Isomers-Definitions Geometrical isomers Nomenclature for Geometrical isomers Optical Isomerism Nomenclature For Optical Isomers Racemic Mixtures 2 Isomers Same molecular formula with different structural formula is called Isomers & phenomenon is called as Isomerism. Example-(C3H8O) H3C CH2 n propanol CH2OH C2H5 O CH3 Methyl ethyl ether 3 Types Of Isomers Stereoisomer Geometrical Isomers Optical Isomers 4 Geometrical Isomers Introduction: Geometrical isomers occur as a result of restricted rotation about a carbon-carbon bond. Single bond between two carbons in a non cyclic structure may rotate around each other at room temperature because as the carbons rotate around each other the degree of overlap between 5 the atomic orbitals is not changed and remain at maximum overlap as rotation occurs. 6 Reason: If there is a double bond between two carbons, these sp2 carbons have a sigma overlap or bond between two sp2 hybrid orbitals and a Pi overlap as a result of the p overlap.The sigma overlap would not change its maximum overlap as a result of rotation about the two sp2 carbons. However if the two sp2 carbons attempt to rotate about each other this will reduce the p-p overlap of the Pi bond. The Pi bond prevents the carbons from rotating about each other. 7 If the similar groups are fixed on the same side as a result of this restricted rotation, then the isomer would be classified as “cis”. The similar groups are fixed due to restricted rotation by double bond on opposite side of the double bond then this isomer is “trans”. The cis and trans isomers are differ in their physical and chemical properties. 8 cis-trans isomers Cis-trans isomers differ from one another in the way the atoms/ groups are positioned in space Cis –same, trans –across They have different physical and chemical properties They interact differently with enzymes/ receptor sites They cannot be interconverted by rotation around C-C bonds Rotation is restricted by double bond or cyclic structure 9 10 e.g H3C CH3 C C H H H3C H C C H CH3 11 E and Z nomenclature rule The E/Z system is based on an assignment of priorities to the atoms or groups attached to each carbon of the double bond If the higher-priority atoms or groups are on opposite sides of the pi bond, the isomer is (E) If the higher-priority groups are on the same side, the isomer is (Z) 12 The atom with the higher atomic number is of higher priority. If two atoms are identical, the atomic numbers of the next atoms are used to assign the priority. Priority is determined at the first point of difference along the chain if the atoms have the same atoms directly attached. 13 H Br H3C H 1 2 ClH2CH 2C Br H3C Cl 3 4 14 On carbon 1, the methyl substituent is of higher priority than H On carbon 2, the bromine substituent is of higher priority than H IUPAC name is (E)-1-bromopropene On carbon 3, the chloroethyl substituent is of higher priority than methyl On carbon 4, the bromine substituent is of higher priority than chlorine IUPAC name is (Z)-1-bromo-1,4-dichloro-2methylbut-1-ene 15 Stereoisomers Same molecular formula - same bond connectivities different arrangement of their atoms in space Optical Isomers non-superimposable mirror images (also called Enantiomers) X W W C C Z Y Y X Z 16 Optical Activity Stereoisomers are said to be optically active if the rotate plane polarized light. Each type of enantiomer rotates light the same amount, but in different directions. Amount and direction of rotation must be experimentally determined using a polarimeter. 17 Plane polarized light Light that has been passed through a nicol prism or other polarizing medium so that all of the vibrations are in the same plane. non-polarized polarized 18 Polarimeter -an instrument used to measure optical activity. polarizer light source analyzer sample tube 19 Chirality Chiral and Achiral Molecules Although everything has a mirror image, mirror images may or may not be superimposable. Some molecules are like hands. Left and right hands are mirror images, but they are not identical, or superimposable. 20 Chirality Chiral: A molecule or object that is not superimposable on its mirror image is said to be chiral. Achiral: A molecule or object that is superimposable on its mirror image is said to be achiral. 21 22 Cont… Chiral Centre : A carbon that is bonded to four different groups of atoms. CH3 * CH CH2 CH3 Cl 2-chlorobutane 23 Enantiomers The enantiomers of lactic acid drawn in two different representations O OH HO O C C C C H CH3 OH HO O O OH OH H CH3 HO OH 24 Enantiomers 3-Chlorocyclohexene Cl Cl 25 Enantiomers A nitrogen chiral center + + N H3 C N CH2 CH3 CH3 CH2 CH3 A pair of enantiomers 26 Diastereomers Mirror plane CH3 H CH3 Cl c c c c OH H H HO CH3 Not mirror images. H Cl CH3 Not mirror images. Not mirror images. CH3 Cl CH 3 H H Cl c c c c OH H CH3 H HO CH3 Nomenclature 1. D & L Nomenclature Dextrorotatory – when the plane of polarized light is rotated in a clockwise direction when viewed through a polarimeter. (+) or (d) Levorotatory – when the plane of polarized light is rotated in a counter-clockwise direction when viewed through a polarimeter. (-) or (l) 28 2. R,S Nomenclature Priority rules 1. Each atom bonded to the chiral center is assigned a priority based on atomic number; the higher the atomic number, the higher the priority (1) (6) (7) (8) (16) (17) (35) (53) -H -CH3 -N H2 - OH - SH - Cl - Br -I Increas ing priority 2. If priority cannot be assigned per the atoms bonded to the chiral center, look to the next set of atoms; priority is assigned at the first point of difference (1) - CH 2 -H (6) - CH 2 -CH 3 (7) - CH 2 -NH2 (8) - CH 2 -OH Increasing priority 29 R,S Nomenclature 3. Atoms participating in a double or triple bond are considered to be bonded to an equivalent number of similar atoms by single bonds. C -CH=CH2 is treated as O -CH is treated as C -CH-CH 2 O C C O H C CH is treated as C C C C H C C 30 Naming Chiral Centers 1. Locate the chiral center, identify its four substituents, and assign priority from 1 (highest) to 4 (lowest) to each substituent. 2. Orient the molecule so that the group of lowest priority (4) is directed away from you. 3. Read the three groups projecting toward you in order from highest (1) to lowest priority (3). 4. If the groups are read clockwise, the configuration is R ; if they are read counterclockwise, the configuration is S. 31 Naming Chiral Centers (S)-2-Chlorobutane 1 H Cl S 2 3 32 Naming Chiral Centers (R)-3-Chlorocyclohexene 3 1 Cl 2 H R (R)-Mevalonic acid 1 1 4 HO CH3 O HO 3 2 R 3 2 OH 33 Racemic mixture an equimolar mixture of two enantiomers because a racemic mixture contains equal numbers of dextrorotatory and levorotatory molecules, its specific rotation is zero. i.e. it is optically inactive. 34 O NH O O O H2C NH O C C N CH2 N S-thalidomide (effective drug) H2C CH2 H O O H O R-thalidomide (dangerous drug) Racemic mixture of Thalidomide 35 Racemic mixture Equal amounts of (+) and (-) enantiomers - rotation is 0 COOH C H 21 []D = +2.6° H3 C OH COOH H C 21 CH3 [] = -2.6° D HO Net rotation is zero. 36 References Stereochemistry of carbon compounds by Ernest L Eliel Stereochemistry of Organic Compounds, Principles and Applications, by D. Nasipuri Stereochemistry Conformation and Mechanism by P.S. Kalsi Organic Chemistry, Sixth Edition, by Robert Thornton Morrison, Robert Neilson Boyd www. google.com 37 38