TOPIC 5: Energetics and Thermodynamics

5.1 MEASURING ENERGY CHANGES
Thermodynamics is the study of energy and how it is interconverted.
First law of thermodynamics – energy cannot be created or destroyed; it can only be converted from one form to another (law of conservation of energy)
Types of energy considered in thermodynamics:
◦ Chemical potential energy (stored in bonds)
◦ Thermal energy (heat)
◦ Kinetic energy (motion of particles)
What is the difference between heat and temperature?
Heat, q, (sometimes referred to as thermal energy) is a form of energy that is transferred from warmer to cooler bodies and has the ability to do work.
As heat is transferred, the result is an increase in the particle movement (kinetic energy).
This change in particle movement is measured as temperature, but sometimes this increase in kinetic energy results in a change of phase instead of a change in temperature.
• In a chemical reaction, we divide the universe into two parts:
• the system (where the reaction takes place) and the surroundings.
• When chemical reactions take place, energy is transferred between the system and the surroundings:
• If energy is released from the system, the surroundings will warm (temperature increases) – this is termed exothermic
• ex: combustion and neutralization reactions
• If energy is absorbed by the system, the surroundings will cool (temperature decreases) – this is termed endothermic
• Because energy is conserved, loss or gain by the surroundings equals the loss or gain by the system.
ENTHALPY
Enthalpy (ΔH measuring in kJ mol -1 ) is the heat transferred by a closed system during a chemical reaction at a constant pressure.
Enthalpy is an example of a state function. A state function is one where any change in value is independent of the pathway between the initial and final measurement. Other examples include volume, temperature, and pressure.
◦ Ex: ΔT = T final
– T initial but this does not account for any heating or cooling that happened between the initial and final measurements of temperature
Enthalpy is measured using a calorimeter. A calorimeter is any apparatus used to measure the amount of heat being exchanged with the surroundings, which is often a solvent such as water.
Chemical reactions that release energy (exothermic) have a negative ΔH.
Chemical reactions that absorb energy (endothermic) have a positive ΔH.
ENTHALPY DIAGRAMS
In an exothermic reaction the enthalpy of the products is lower than that of the reactants.
In an endothermic reaction the enthalpy of the products is higher than that of the reactants.
The products are described as being energetically more stable than the reactants.
The products are described as being energetically less stable than the reactants.
ENTHALPY
To calculate ΔH we need to find the heat change. When calculating the heat change of a pure substance, we need the specific heat capacity, c, measured in kJ∙kg -1 ∙K -1 .
◦ The specific heat capacity is measure of how much energy is need to raise 1 kg of the substance 1 o C or 1 K.
Heat, q, can be calculated using: q = mcΔT
When adding energy to a substance, a low specific heat capacity will result in a higher rise in temperature compared to adding the same energy to a substance with a higher specific heat capacity
Specific heat capacity of copper:
0.385 J∙g -1 ∙K -1
Specific heat capacity of ethanol:
2.44 J∙g -1 ∙K -1
Practice #1:
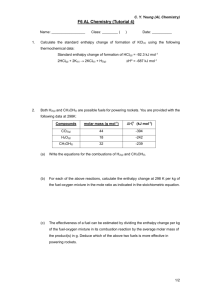
When a 1.15 g sample of anhydrous lithium chloride, LiCl, was added to 25.0 g of water in a coffee-cup calorimeter, a temperature rise of 3.80 K was recorded. Calculate the enthalpy change of solution for 1 mol of lithium chloride.
ENTHALPY CHANGE OF FORMATION
The enthalpy change of a reaction can be determined using:
ΔH Θ reaction = ∑(ΔH f
Θ products) - ∑(ΔH f
Θ reactants)
ΔH f
Θ is the standard enthalpy change of formation of a substance. This is the energy change upon the formation of 1 mol of a substance from its constituent elements in their standard state.
◦ The value and sign of the calculated enthalpy of formation informs us about the energetics of the reaction.
◦ Ex: The enthalpy of formation for methane is:
C(s) + 2H
2
(g) CH
4
(g) ΔH f
Θ = -74.9 kJ mol -1
◦ Ex: The enthalpy of formation for phenol is:
6C(s) + 3H
2
(g) + ½O2(g) C
6
H
5
OH(s)
NOTE: ΔH f
Θ for elements (including diatomics) is 0 kJ mol -1 .
ΔH f
Θ = -165.0 kJ mol -1
Practice #2:
Write equations to describe the standard enthalpy change of formation for the following compounds and state the enthalpy value by referring to the Data Booklet: a) Propane b) Chloromethane c) Ethanol d) Benzoic acid e) Carbon monoxide f) Methylamine
ENTHALPY CHANGE OF COMBUSTION
The enthalpy change of combustion ΔH c
Θ is the heat evolved upon the complete combustion of 1 mol of substance.
Ex: Butane is highly flammable:
C
4
H
10
(g) + 13/2 O
2
(g) 4CO
2
(g) + 5H
2
O(l) ΔH c
Θ = -2878 kJ mol -1
Can also be written so that ΔH f
Θ is included in the thermochemical equation:
C
4
H
10
(g) + 13/2 O
2
(g) 4CO
2
(g) + 5H
2
O(l) + 2878 kJ mol -1
Ex: Benzene, C6H6, is highly flammable, producing a sooty flame:
2C
ΔH
6
Θ
H
6
(l) + 15O
2
(g) 12CO reaction = ∑(ΔH f
Θ
2
(g) + 6H
2
O(l) products) - ∑(ΔH f
Θ reactants)
= [12 x (-393.5) + 6 x (-285.5) – 2 x (+49.0) – 15 x 0] kJ
= (-4722 – 1714.8 – 98.0) kJ
= -6535 kJ
Practice #3:
Determine the standard enthalpy of formation for photosynthesis. Compare this to the value found in section 13 of the Data Booklet. (The ΔH f
Θ for glucose is -1273.3 kJ mol -1 .)
6CO
2
(g) + 6H
2
O(l) C
6
H
12
O
6
(aq) + 6O
2
(g)
5.2 HESS’S LAW
Since enthalpy is a state function, Hess’s law can be used to determine the total enthalpy change through a reaction. Most reaction usually show the net reaction, which is a summary of different reactions, which when added together give the overall reaction.
Hess’s law states that regardless of the route by which a chemical reaction proceeds, the enthalpy change will always be the same providing the initial and final states of the system are the same.
Summing the individual reactions (taking into
account direction and magnitude), results in an overall reaction.
Two methods to solve Hess’s Law for ΔH f
Θ :
◦ Summing of equations
◦ Enthalpy cycle
Practice #4:
Calculate the ΔH for the reaction: C
2
H
4
(g) + H
2
(g) C
2
H
6
(g), from the following data.
C
2
H
4(g)
+ 3O
2
2CO
2(g)
+ 2H
2
O
(l)
2C
2
H
6(g)
+ 7O
2(g)
4CO
2(g)
+ 6H
2
O
(l)
2H
2(g)
+ O
2(g)
2H
2
O
(l)
ΔH = -1411. kJ
ΔH = -1560. kJ
ΔH = -285.8 kJ
5.3 BOND ENTHALPY
What does enthalpy really measure? Every bond that is broken required energy to do so. The energy required to break one mole of bonds in a gaseous covalent
molecule under standard conditions is called bond enthalpy. Bond breaking is an endothermic process which is a positive enthalpy value.
◦ Ex: H
2
(g) 2H (g) ΔH Θ = +436 kJ mol -1
Bond enthalpy is also referred to as bond dissociation enthalpy. These are average values based on experimental data.
Bond enthalpy can be calculated using following equation and data from the data booklet (DB section 11):
ΔH Θ = ∑(BE bonds broken) - ∑(BE bonds formed)
Factors that affect bond enthalpy:
1. Bond length. Think of the hydrogen halides (HF, HCl, HBr,
HI). As you go down the family, the ionic radius increases, which means the bond length increases. Longer bond = weaker bond = less energy to break it.
2. Bond strength. As you increase the number of electrons in a bond (single, double, and triple), the strength increases.
More electrons in the bond = more energy needed to break it.
3. Bond polarity. As the polarity of a bond increases, the more ionic character a molecule has (stronger dipoles, partial charges). The partial charges attract each other, making the bond stronger. More bond polarity = stronger bond = more energy required to break it.
Bond
H-F
H-Cl
H-Br
Bond
C-C
C=C
C≡C
Bond
H-F
H-Cl
H-Br
Length
(pm)
92
128
141
BE
(kJ/mol)
567
431
366
Length
(pm)
154
134
120
BE
(kJ/mol)
346
614
839
Δχ
P
1.8
1.0
0.8
BE
(kJ/mol)
567
431
366
Practice #5:
Find the enthalpy change for the following reaction using section 11 of the data booklet.
C
2
H
4
(g) + HBr(g) C
2
H
5
Br(g)
Practice #6:
Calculate the ΔH c
Θ for the complete combustion of octane, C enthalpy values in the DB.
8
H
18
, using the bond
2C
8
H
18
(g) + 25O
2
(g) 16CO
2
(g) + 18H
2
O(g)
Compare the value you calculated to the ΔH c
Θ calculated using the data from
Section 13: -5470 kJ mol -1 . Explain the difference.
5.3 BOND ENTHALPY - OZONE
Ozone, O
3
, is both created and destroyed in the stratosphere layer of Earth’s atmosphere. UV rays from the sun split O
2 atoms are free to bond with O
2 into single O atoms. These single O molecules forming O
3
.
O
2
(g) –UV O ∙ (g) + O ∙ (g) O
2
(g) + O ∙ (g) O
3
(g)
Ozone is very effective at absorbing harmful long- and short-wavelength UV radiation. This absorption breaks down ozone into O
2 and O again. Without ozone, UV radiation would damage cells of all the living creatures.
BE for O-O in O
2
BE for O-O in O
3
= 498 kJ mol -1
= 364 kJ mol -1
Chlorofluorocarbons (CFCs) are a type of hydrocarbon containing carbon, hydrogen, and halogen atoms. The compounds themselves are considered non-toxic and have low level of flammability and reactivity. This, however, is only true at the Earth’s surface. As they rise into the atmosphere, the UV radiation cleaves a chlorine atom from the molecule.
This chlorine has a catalytic effect on the destruction of ozone. Since the chlorine is not consumed, a small amount of CFC can destroy a large quantity of ozone.
Cl ∙ (g) + O
3
(g) ClO ∙ (g) + O
ClO ∙ (g) + O
3
2
(g) Cl ∙ (g) + 2O
(g)
2
(g)
Since the 1920s, the use of CFCs (particularly Freon) has increased dramatically in refrigeration, air conditioning, and the automobile industry. In the
1980s, scientists began to monitor the depletion of the ozone layer. Since CFCs have had a massive economic (positive) and environmental (negative) impact, it has been the focus of many discussions.