rate

Chapter 14
Chemical Kinetics
Review Section of Chapter 14 Test
• Net Ionic Equations
Reaction Rate
• The rate of a chemical reaction is measured as the decrease in the concentration of a reactant or the increase in the concentration of a product in a unit of time.
Reaction Rate
• The rate of a chemical reaction is measured as the decrease in the concentration of a reactant or the increase in the concentration of a product in a unit of time.
Rate =
∆[ ]
∆time
Reaction Rate
• The rate of a chemical reaction is measured as the decrease in the concentration of a reactant or the increase in the concentration of a product in a unit of time.
Rate =
∆[ ]
∆time
What units would we use for rate?
Reaction Rate
• The rate of a chemical reaction is measured as the decrease in the concentration of a reactant or the increase in the concentration of a product in a unit of time.
Rate =
∆[ ]
∆time
2H
2
O
2
(aq) → 2H
2
O(l) + O
2
(g)
Reaction Rate
• The rate of a chemical reaction is measured as the decrease in the concentration of a reactant or the increase in the concentration of a product in a unit of time.
Rate =
∆[ ]
∆time
2H
2
O
2
(aq) → 2H
2
O(l) + O
2
(g)
How could the rate be expressed for this reaction in terms of H
2
O
2
?
2H
2
O
2
(aq) → 2H
2
O(l) + O
2
(g)
2H
2
O
2
(aq) → 2H
2
O(l) + O
2
(g)
2H
2
O
2
(aq) → 2H
2
O(l) + O
2
(g)
What is the rate of the reaction from 0s to 2.16 x 10 4 s?
2H
2
O
2
(aq) → 2H
2
O(l) + O
2
(g)
What is the average rate of appearance of O
2 from 0s to 2.16 x 10 4 s?
General Rate of Reaction
a A + b B → c C + d D
Rate of reaction = rate of disappearance of reactants or
Rate of reaction = rate of appearance (formation) of products
We can use the coefficients in the equation to compare the reaction rates for all the substances in the reaction.
15-1 The Rate of a Chemical
Reaction
• Rate is change of concentration with time.
2 Fe 3+ (aq) + Sn 2+ (aq) → 2 Fe 2+ (aq) + Sn 4+ (aq) t = 38.5 s [Fe 2+ ] = 0.0010 M
∆t = 38.5 s ∆
[Fe 2+ ] = (0.0010 – 0) M
Δ[Fe 2+ ]
Rate of formation of Fe 2+ = = = 2.6 x 10 -5 M s -1
Δt
0.0010 M
38.5 s
Rates of Chemical Reaction
2 Fe 3+ (aq) + Sn 2+ (aq) → 2 Fe 2+ (aq) + Sn 4+ (aq)
Rate of formation of Fe 2+ = 2.6 x 10 -5 mol L -1 s -1
What is the rate of formation of Sn 4+ ?
What is the rate of disappearance of Fe 3+ ?
What does the slope of the line represent?
What is the concentration at 100s for the reaction:
2H
2
O
2
(aq) → 2H
2
O(l) + O
2
(g)?
[H
2
O
2
] i
= 2.32 M
Given:
Rate = 1.7 x 10 -3 M s -1 =
- Δ[H
2
O
2
]
Δt
-Δ[H
2
O
2
] = (1.7 x 10 -3 M s -1 ) (∆t)
∆[H
2
O
2
] = -(1.7 x 10 -3 M s -1 )(100 s) = -0.17M
[H
2
O
2
]
100 s
= 2.32 M - 0.17 M
= 2.15 M
What does it mean when the rate of a reaction reaches zero?
• For a normal reaction it means that one or more of the reactants are used up and the reaction has stopped.
• For a reversible reaction it means that the reaction has reached equilibrium .
Factors Affecting Reaction Rates
1. The nature of the reacting substances.
Factors Affecting Reaction Rates
2. The state of subdivision of the reacting substances.
Factors Affecting Reaction Rates
3. The temperature of the reacting substances.
Factors Affecting Reaction Rates
4. The concentration of the reacting substances.
Air (21% oxygen) 100% oxygen
Factors Affecting Reaction Rates
5.
The presence of a catalyst.
Catalysts speed up reactions but are left unchanged by the reaction.
The Rate Law
a A + b B
….
…. → g G + h H
Rate = k [A] m [B] n
….
Rate constant = k (k is constant for a particular reaction at a specific temperature)
Order of A = m Order of B = n
Overall order of reaction = m + n +
….
Temperature and Rate
• Generally, as temperature increases, so does the reaction rate.
• This is because k is temperature dependent.
Use the data provided to write the rate law and indicate the order of the reaction with respect to HgCl the overall order of the reaction.
2 and C
2
O
4
2and also
First determine the order of HgCl
2
Next determine the order of C
2
O
4
2-
Now write the rate law and determine the order of the reaction.
Calculate the rate constant “k” and its units.
Initial rate of disappearance HgCl
2 mol L -1 min -1
What is the average rate of disappearance of C
2
O
4
2in trial 1?
Initial rate of disappearance HgCl
2 mol L -1 min -1
Use the data provided to write the rate law and indicate the order of the reaction with respect to NO
2 and CO (support your answers). Also give the overall order of the reaction.
( Example is in notebook )
Calculate the rate constant “k” and its units.
What is the average rate of disappearance of CO in trial 2?
Concentration and Rate Summary
•
After finding the trials to compare:
• A reaction is zero order in a reactant if the change in concentration of that reactant produces no effect on the rate.
• A reaction is first order if doubling the concentration causes the rate to double.
• A reaction is n th order if doubling the concentration causes an 2 n increase in rate.
• Note that the rate constant does not depend on concentration.
Collision Model
•Key Idea : Molecules must collide to react.
•However, only a small fraction of collisions produces a reaction. Why?
Two Factors
Collisions must have enough energy to produce the reaction (must equal or exceed the activation energy ).
Orientation of reactants must allow formation of new bonds.
2HI → H
2
+ 2I
Concentration and Collision Theory
• Why does an increase in concentration cause an increase in reaction rate?
Concentration and Collision Theory
• Why does an increase in concentration cause an increase in reaction rate?
• Increasing the concentration increases the number of collisions and therefore there are more collisions leading to product.
Temperature and Collision Theory
• Why does a temperature increase cause the reaction rate to increase?
Temperature and Collision Theory
• Why does a temperature increase cause the reaction rate to increase?
• At higher temperatures there are more collisions and a greater percentage of the collisions have the energy necessary to create a successful collision.
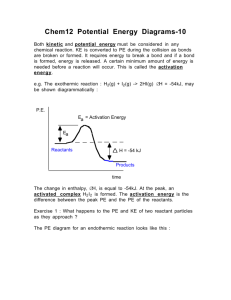
Activation Energy
• The activation energy is the minimum amount of energy necessary for a reaction to occur.
Temperature and Activation Energy (E a
)
Figure 14.12 (Page 432)
Activation Energy
• The activation energy can also be thought of as the energy necessary to form an activated complex during a collision between reactants.
Transition State Theory
• The activated complex is a hypothetical species lying between reactants and products at a point on the reaction profile called the transition state .
The activated complex is a transition state between reactants and products where old bonds have begun to break and new bonds have started to form. It cannot be isolated.
Determining the Activation Energy
“The Arrhenius Equation”
- Collisions must have enough energy to produce the reaction (must equal or exceed the activation energy).
- Orientation of reactants must allow formation of new bonds.
Arrhenius Equation
k Ae
A
• k = rate constant
• A = frequency factor
• E a
= activation energy
• T = temperature
• R = ideal gas constant frequency factor : a value in the Arrhenius equation indicating how many collisions have the correct orientation to lead to products.
Arrhenius Equation
Determination of Activation Energy
• Graphical determination of activation energy (E a
).
– plot the ln k on the y-axis.
– Plot 1/ T (use Kelvin temperature) on the x-axis.
Arrhenius Equation
Determination of Activation Energy
• A plot of ln k versus 1/ T (using Kelvin) will have:
– slope of – Ea/R
– y-intercept of ln A .
ln A
Slope = -
E a
R
Example 14.12 Page 433
Example 14.12 Page 433
x-axis y-axis
Example 14.12 Page 433
x
1
,y
1
= 1.25 x 10 -3 , -2.593
x
2
,y
2
= 1.78 x 10 -3 , -14.447
Example 14.12 Page 433
For two reactions at the same temperature, the reaction with the higher activation energy has the lower rate constant (k) and the slower rate.
2O
3
3O
2
- A chemical equation like the one above does not tell us how reactants become products - it is simply a summary of the overall reaction.
The reaction: 2O
3
3O
2
- Is proposed to occur through the two step process given below:
O
3
O
3
O
2
+ O
+ O
2O
2
This two step process is an example of a reaction mechanism
Reaction Mechanisms
• A reaction mechanism is a step-by-step description of a chemical reaction.
• Each step is called an elementary reaction .
Often Used Terms
•Intermediate : formed in one step and used up in a subsequent step and so is never seen as a product.
•Molecularity : the number of species that must collide to produce the reaction indicated by that step.
•Elementary Step : A step within a reaction mechanism whose rate law can be written from its molecularity.
Reaction Mechanisms
Elementary Steps
• Molecularity
: the number of molecules present in an elementary step.
– Unimolecular: one molecule in the elementary step,
– Bimolecular: two molecules in the elementary step, and
– Termolecular: three molecules in the elementary step.
• It is not common to see termolecular processes
(statistically improbable).
Reaction Mechanisms
Rate Laws for Elementary Steps
• The rate law of an elementary step is determined by its molecularity:
– Unimolecular processes are first order,
– Bimolecular processes are second order, and
– Termolecular processes are third order.
Reaction Mechanisms
Rate Laws for Elementary Steps
The Rate Determining Step
Rate-Determining Step
•In a reaction mechanism, the rate determining step is the slowest step. It therefore determines the rate of reaction.
Reaction Mechanisms
• Reaction mechanisms must be consistent with:
1.Stoichiometry for the overall reaction.
2.The experimentally determined rate law.
NO
2
(g) + CO(g)
NO(g) + CO
2
(g)
Reaction mechanism must be consistent with the stoichiometry of the overall reaction.
•
Is the mechanism below consistent with the overall reaction above?
NO
2
(g) + NO
2
(g)
NO
3
(g) + NO(g)
NO
3
(g) + CO(g)
NO
2
(g) + CO
2
(g)
Determining the stoichiometry of a reaction mechanism.
Page 439
Reaction Mechanisms
• The reaction mechanism must also support the rate law.
Reaction Mechanisms
Rate Laws for Multistep Mechanisms with an initial fast step.
• Consider the reaction:
2NO( g ) + Br
2
( g )
2NOBr( g )
Reaction Mechanisms
Mechanisms with an Initial Fast Step
2NO( g ) + Br
2
( g )
2NOBr( g )
• The experimentally determined rate law is
Rate = k [NO] 2 [Br
2
]
• Consider the following mechanism k
Step 1: NO(g) + Br
2
(g) NOBr
2
(g) k
-1
(fast)
Step 2: NOBr
2 k
(g) + NO(g) 2NOBr(g) (slow)
Step 1: NO(g) + Br
2 k
(g) NOBr
2
(g) k
-1
Step 2: NOBr
2 k
(g) + NO(g) 2NOBr(g)
(fast)
(slow)
• The rate law is (based on
Step 2 ):
Rate = k
2
[NOBr
2
][NO]
• The rate law should not depend on the concentration of an intermediate (intermediates are usually unstable).
• NOBr
2 is an unstable intermediate, so we express the concentration of NOBr
2 in terms of NOBr and Br
Since there is an equilibrium in step 1 we have
2
[ NOBr
2
]
k
1 k
1
[ NO][Br
2
]
Step 1: NO(g) + Br
2 k
(g) NOBr
2
(g) k
-1
Step 2: NOBr
2 k
(g) + NO(g) 2NOBr(g)
(fast)
(slow)
• By definition of equilibrium: k
1
[ NO][Br
2
]
k
1
[ NOBr
2
]
• Therefore, the overall rate law becomes
Rate
k
2 k
1 k
1
[ NO][Br
2
] [ NO]
k
2 k
1 k
1
[ NO]
2
[Br
2
]
• Note the final rate law is consistent with the experimentally observed rate law.
Determining the rate law of a reaction mechanism.
Page 439
Catalysts
• A catalyst is a substance that increases the rate of a chemical reaction by reducing the activation energy, but which is left unchanged by the reaction.
What is the overall reaction?
O
3
O
3
O
2
+ O
+ O
2O
2
What is the overall reaction?
Identify the intermediates.
Identify the intermediates.
NO is a catalyst
A homogeneous catalyst is of the same phase as the reacting substances. It lowers the activation energy by forming intermediates which allow the reaction to proceed by a different pathway.
Heterogeneous Catalysts
• A heterogeneous catalyst is of a different phase than the reacting substances. It lowers the activation energy by providing a surface on which the reaction can occur.
Inhibitor
• An inhibitor decreases the rate of a reaction. It often does this by rendering a catalyst ineffective.
Catalyst “poisoning” occurs when a catalytic converter is exposed to exhaust containing substances that coat the working surfaces, encapsulating the catalyst so that it cannot contact and treat the exhaust. The most notable contaminant is lead, so vehicles equipped with catalytic converters can only be run on unleaded gasoline.
Inhibitor
• An inhibitor decreases the rate of a reaction.