Practical Pain Management - Idaho Quality of Life Coalition
advertisement

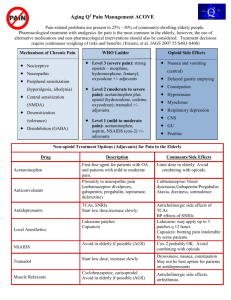
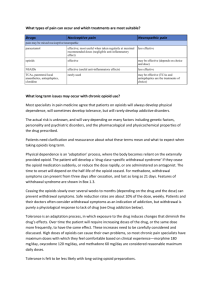
Pain ManagementDrug Therapy Presented by Robert A. Ancker, MD Medical Director, Hospice of North Idaho Module Objectives Review the role of acetaminophen and NSAIDS in pain management Learn the difference between Psychological vs. Physiological Dependence Learn the key pharmacological principles of opioid analgesics and proper usage Learn the key opioid side effects Learn the indications and use of Relistor ® for Opioid Induced Constipation Pain Assessment 1. Basic History – – – – When did it start? Where is it? What is the pain intensity? 0-10 scale What does it feel like? (quality) • • • – Somatic – localized, achy, dull Visceral – colicky, pressure, referred, diffuse Neuropathic – burn, numb, radiate, hyperalgesia What makes the pain better or worse? 2. Analgesic History—Medications 3. Analgesic History: Non-Pharmacological 4. Impact and Meaning of Pain 5. Pain Causality and Patient Goals Visual Analog Scale (VAS) Psychological vs. Physiological Dependence Psychological Dependence (Addiction) – Compulsive use of drugs – Loss of control – Use in spite of harm – Occurs in only ~0.1% of chronic opioid patients – Drug seeking behavior: missed appointments, off-hours calls for renewals, multiple physicians Physiological Dependence (Tolerance) – Physical withdrawal Sx on abrupt discontinuation – Expect after 1 month of opioid use – Long term use results in tolerance & thus increased doses (NORMAL) – Usually does not occur during acute pain management – Risk of MD directed addiction is NOT a concern in treating cancer pain Tolerance and Dependence Tolerance is not an inevitable consequence of chronic opioids therapy– RARE in cancer patients – Increased dose correlates with disease progression Physical dependence is expected with chronic therapy Do not confuse physical dependence with ADDICTION (psychological dependence) *Drayer et al. J Pain Symptom Manage 1999;17:434-40 Beware of the Pseudoaddict!! Pseudoaddiction is .. The behavioral manifestations of addiction that occur as a result of under treated pain – – – – Moaning/crying when you enter the room Clock watching Frequent requests for more medication Pain that seems “excessive” for the stimulus Patient has no other history to suggest addiction – Behaviors cease with adequate pain treatment Pseudoaddiction May occur in the hospitalized patient, in pain, who has opioids ordered: – At inadequate potency or dose – At excessive dosing intervals – And when the behavior is reinforced by MD or RN behavior that tends to limit opioid use: “you really shouldn’t be having this much pain” “you have to wait another two hours for your next dose of medication” Checkpoint Pain Type: Somatic – localized, achy, dull Visceral – colicky, pressure, referred, diffuse Neuropathic – burn, numb, radiate, hyperalgesia Classes of Analgesics Non-opioids (NSAIDS, acetaminophen, aspirin, tramadol) Opioids (morphine is the prototype) Adjuvant Analgesics (antidepressants, anticonvulsants, steroids, others) Analgesics for Mild Pain Acetaminophen NSAIDS/Aspirin Tramadol Acetaminophen Why is Tylenol, the pain reliever recommended by most doctors? Doesn’t affect stomach, kidney, platelets – But, it has no inflammatory mediators – But, it has a ceiling dose: 4 Grams/24 hours – AND, is hepatotoxic in overdose Checkpoint What is the dose of acetaminophen (APAP) in the following preparations: – Lorcet Plus ® = 650 mg APAP – Vicodin ES ® = 750 mg APAP – Ultracet ® = 325 mg APAP – Norco ® = 325 mg APAP Acetaminophen toxicity Acetaminophen overdose is one the most common causes of OTC drug poisoning in the United States. More than 78,000 ER cases w/ 33,000 hospital admits per year of acetaminophen overdoses per report by the Consumer Products Safety Commission About 300 deaths per year 50/50 accidental to intentional Leading cause of drug-induced liver failure in the United States (Bartlett D 2004). Bartlett D. Acetaminophen toxicity. J Emerg Nurs. 2004 Jun;30(3):281–3. NSAIDs Effective for relief of mild pain & have opioid dose-sparing effect Pain relief via their anti-inflammatory effect by inhibiting prostaglandins, thus are very effective in relieving pain from bone mets. Dosage: use patient response to determine effective dose If max. dose achieved w/o pain relief try another drug from this same category Route of Admin.: Use oral route; some suppositories available; Toradol via IM or IV NSAIDs - toxicity Has significant end organ toxicity – Should be used with great caution in the elderly – ~16,500 deaths, ~100,000 hospitalizations annually due to adverse effects from NSAIDS – ~3500 deaths from GI bleed Note! No NSAID has greater analgesic efficacy compared to any other NSAID – However, patients may report greater effect from a particular preparation NSAIDs - toxicity Gastropathy Renal insufficiency Decreased platelet aggregation Hypersensitivity Hepatitis with some 2nd Generation NSAID’s reported to have less GI toxicity – Maybe?? – Vioxx® fiasco…… Tramadol (Ultram ®) A synthetic non-opioid(?) analog of codeine – mu-receptor agonist – Weak inhibitor of serotonin, norepinephrine reuptake Analgesic effect roughly equivalent to Tylenol #3 ® – Efficacy variable; has an analgesic ceiling No anti-inflammatory effects Side effects similar to opioids at high dose-nausea, confusion, dizziness, constipation Seizure risk Dependence may occur Not currently on a DEA schedule! PDR.net. 2003: Ultram® (tramadol hydrochloride). Cherny NI. Opioid analgesics. Comparative features and prescribing guidelines. Drugs. 1996;51:714-37. Opioid Pharmacology Opioid refers to – Alkaloids derived from opium – Natural and synthetic agents whose actions are mediated by selectively binding to opioid receptors in the brain/spinal cord and perhaps via peripheral opioid receptors Receptor types include mu, delta, & kappa – Further divided into receptor subtypes, eg: mu1 & mu2 – The mu receptor is the dominant analgesic receptor, but other receptors play a role in analgesia for certain opioids – There is no dose ceiling for pure agonists opioids, only for acetaminophen in combination products Cherny NI. Drugs. 1996; 51:714-37. Responses Mediated by Opioid Receptors Receptor mu Response on activation Analgesia, respiratory depression, miosis, euphoria, reduced GI motility delta Analgesia kappa Analgesia, dysphoria, psychotomimetic effects, miosis, respiratory depression Adapted from: Cherny NI. Drugs. 1996; 51:714-37. Mu Receptor Activation of Opioids morphine mu receptor site FULL AGONIST activation of mu receptor Stadol ® mu receptor site PARTIAL AGONIST activation of mu receptor naloxone mu receptor site ANTAGONIST - Prevents or reverses activation of mu receptor Zuurmond WW, et al. Acta Anaesth Belg. 2002;53:196-201. Cherny NI. Drugs. 1996; 51:714-37. Walsh SL, et al. Clin Pharmacol Ther. 1994;55:569-80. Mixed Agonist-Antagonists Claim to have less respiratory depressant effects—not substantiated Claim to be less addicting—not substantiated Will potentiate withdrawal in patients being treated with pure agonists – NEVER administer to a patient on a pure agonist Have an analgesic ceiling Are psychotomimetic – can cause psychoses No real indication in end of life patients Opioid pharmacology Cmax after – PO 1 hr – SC, IM 30 min – IV 6 min Half-life at steady-state – PO/PR/SC/IM/IV 3-4 hrs . . . Opioid pharmacology Steady state after 4-5 half-lives – Steady state after 1 day (24 hours) Duration of effect of “immediaterelease” formulations (except methadone) – 3-5 hours PO/PR – Shorter with parenteral bolus Plasma Concentration IV SC / IM Cmax 0 po / pr Half-life (t1/2) Time OPIOIDS—Duration of Action A. Ultra short B. Short C. Long A. Ultra Short Acting Opioid Fentanyl – IV has 50-100 x potency of morphine – transdermal – Transmucosal Good role in Renal and/or Liver failure patients B. Short Acting Opioids Parenteral or Oral – morphine – hydromorphone (Dilaudid ®) – meperidine (Demerol ®) – codeine Oral only – oxycodone (Percocet ®, Tylox ® , OxyIR) – hydrocodone (Vicodin ® Lortab ®, Norco ®) – propoxyphene (Darvon ®, Wygesic ®) – Note: hydrocodone is only available as a combination product. B. Short Acting Opioids Oral dosing: – – – – onset in 20-30 min peak effect in 60-90 minutes duration of effect 2-4 hours Can be dose escalated or re-administered every 2-4 hours for poorly controlled pain as long as the daily Acetaminophen dose stays < 4 grams Opioid Combination Products Opioids available as combination products with acetaminophen or aspirin • Codeine; hydrocodone; oxycodone; Typically used for • Moderate episodic (PRN) pain – UOOB, BSC, ROM – May not need any med OR need PRE-MED • Breakthrough pain in addition to a long-acting opioid – KEEP TRACK Never prescribe more than one combination drug at any one time How to Choose a Combination Product (cont) Toxicity: – – – All the combination products can cause opioid toxicities: nausea, sedation, constipation, etc. There is little published data that supports the use of one product over another in terms of toxicity; however … codeine is probably the most emetogenic opioid Meperidine Shortest acting (only 2-3 hr duration) Weak potency; – 300 mg po = 30 mg po morphine Converted to a long acting toxic metabolite--a CNS stimulant – Tremor, myoclonus and seizure – Risk highest with prolonged use and renal insufficiency C. Long Acting Opioids Oral – – – – MS Contin® Oramorph SR® Oxycontin® methadone Transdermal – Fentanyl Patch (Duragesic®) MS Contin® / Oxycontin® No clear benefit of one product over another – MS Contin contains morphine – Oxycontin contains oxycodone – No difference in toxicity; No difference in addiction potential All must be taken intact—they cannot be crushed; they do not fit down GI tubes. – Exception: Kadian ® All provide 8-12 hours of analgesia – Minimum dosing interval is q 8 hours – All provide onset of analgesia within 2 hours – All can be dose escalated every 24 hours Transdermal Fentanyl Slow onset of action: 13-24 hours – Duration of action: 48-72 hours Should only dose escalate q 3 days – Fentanyl stays in circulation for up to 24 hours after patch removal Place on hairless, non-irradiated skin No ceiling dose Conversions from/to Transdermal Fentanyl (Duragesic®) When converting fentanyl patches, published data suggest that a 25 mg patch is equivalent to 45-135 mg of oral morphine/24h. However, clinical experience suggests that most patients will use the lower end of the range of morphine doses (i.e., for most patients 25 mcg is ≈ 50 mg of oral morphine/24h). THUS, 24 hour total dose of oral morphine, divided by 2 = dose in micrograms of transdermal fentanyl; Conversions from/to Transdermal Fentanyl (Duragesic®) Example: • MS Contin® 30 mg q 12 = 60 mg MS/24 hours • 60 divided by 2 = 30; rounded to one 25 ug Fentanyl Patch Example: • Duragesic 50 mcg/hr = ~100 mg oral morphine per day Breakthrough Pain Patients on any long-acting med always need a second, short-acting med, available for breakthrough pain Take at least every 4 hours, preferably less Guideline, dose of breakthrough opioid should be: 10-20% of 24 hour dose of analgesics and made available q. 1-4 hours Methadone Complex pharmacology Duration of action increases with prolonged use from 4 hours to as much as 12 hours Dose conversions to:from other opioids are complex—seek consultation Least expensive potent opioid Does not need special DEA license to use for pain; special license only needed if used as treat for substance abuse (methadone maintenance clinic) Opioid Dose Escalation Always increase by a percentage of the present dose based upon patient’s pain rating and current assessment 50-100% increase 25-50% increase 25% increase Mild pain 1-3/10 Moderate pain 4-6/10 Severe pain 7-10/10 Frequency of dose escalation The frequency of dose escalation (oral opioids) depends on the particular opioid – Short acting oral: q 2-4 – Long acting oral, except methadone: q 24 hours – Methadone: q 4-6 DAYS – Transdermal fentanyl: q 72 hours Parenteral Opioids IV is the route of choice if access is available – There is no indication for IM opioids – All standard opioids can be given SQ, by either bolus dose or by continuous infusion Can use a SQ button – Change 2x/week – Limit 3ml/hr PCA (basal rate plus a patient initiated dose) is an effective and well accepted modality; either IV or SQ Parenteral Opioids IV or SQ bolus doses have a shorter duration of action that oral doses; typically 1-3 hours The peak effect from an IV bolus dose is 5-15 minutes Dose escalation of parenteral opioids is the same as with oral—always by a percentage of the starting dose Analgesic drug concentration Theoretical Relation Between Analgesic Drug Level, Dosing Interval, and Clinical Response IM PCA Sedation Dose Analgesia Minimum analgesic concentration Dose Dose 6 8 Dose 10 12 Time (hours) IM=intramuscular; PCA=patient controlled analgesia Ferrante FM, et al. Anesth Analg. 1988;67:457-61. Pain 14 Equianalgesia Since all potent opioids produce analgesia by the same pharmacological mechanism, they will produce the same degree of analgesia if provided in equianalgesic doses. Thus, there is little basis to say, “morphine did not work, but hydromorphone did work”. Such a statement generally means that nonequianalgesic doses were used. There is no dose ceiling. The word “potent” is irrelevant! Non-opioids are not included when performing equianalgesic calculations. Equianalgesia Common Conversions 10 po MS = 3.3 mg IV MS (3:1) 5 mg po Dilaudid = 1 mg IV Dilaudid (5:1) 10 mg po MS = 2.5 mg po Dilaudid® (4:1) 10 mg po MS = 10 mg po oxycodone ** 10 mg po MS = 10 mg po hydrocodone (!) Note: Conversions factors are only a rough guide to approximate the correct dose. ** Controversial: some recommend 3:2 or 1.5:1 ratios for MS: oxycodone Incomplete Cross-tolerance If a switch is being made from one opioid to another because of tolerance, it is recommended to start the new opioid at 50% of the equianalgesic dose Opioids Side Effects Sedation, confusion, respiratory depression Dizziness, dysphoria Nausea Constipation Itching, uticaria, bronchospasm – NOT AN ALLERGY!!! Urinary retention Opioid hyperexcitability syndrome – Hyperesthesia, myoclonus, seizure Sedation / Respiratory Depression With increasing dose, all opioids lead to a predictable sequence of CNS events: – Sedation with or without delirium then • Further decrease consciousness then – Coma and respiratory depression Respiratory Depression Risk Factors – Renal insufficiency – Liver failure – Parenteral opioids; especially rapid dose escalation in opioid naïve patients – Severe pulmonary disease (CO2 retainers) – Sleep apnea – Rapid dose escalation of transdermal fentanyl or methadone Naloxone (Narcan®) In palliative care, Narcan is only indicated when: – **The goals of care are such that reversing CNS depression is indicated** • ?? Intentional/accidental overdose – And patients have decreased level of consciousness and decreased respirations – Administer Narcan • 1 amp (0.4 mg) diluted in 9 ml saline • 1 ml per minute until level of consciousness improves – CAUTION: may propagate a severe pain crisis! Nausea and Vomiting Caused by stimulation of the CTZ (chemoreceptor trigger zone) at base of 4th ventricle. – Nausea is not an allergy!! Morphine and codeine are the most emetogenic opioids Tolerance develops within 3-7 days for most patients Standard anti-emetics can reduce symptoms Itching and Urticaria Tolerance may or may not develop. Not life threatening – not anaphylaxis, NOT AN ALLERGY! – does not mean that opioids can never be used Drug treatment of symptoms is not very effective (anti-histamines, steroids) – Trial of different opioids indicated as some patients will itch with one product but not another Constipation Multi-factorial cause Tolerance does not develop Start a bowel stimulant at the time opioids are started – Senna or MOM good first choices Goal is one BM qod, or what is “normal” for the patient Relistor SQ Methylnaltrexone Bromide Relistor ® 12mg/0.6ml solution for SQ injection Etiology of Opioid-Induced Constipation (OIC) Opioids: suppress forward peristalsis raise sphincter tone increase fluid absorption reduce intestinal secretions Largely mediated by peripheral mu ()-opioid receptors: on myenteric and submucosal neurons in the gut Gutstein HB, Akil H. Opioid Analgesics. Goodman and Gillman’s: The Pharmacological Basis of Therapeutics. 11th ed. New York: NY: McGraw-Hill; 2006. Schumacher MA, Basbaum AI, Way WL. Opioid analgesics & antagonists. Basic and Clinical Pharmacology. 10th ed. New York, NY: Lange Medical Books/McGraw-Hill; 2007. Methylnaltrexone: Acts peripherally to directly counteract the constipating effects of opioid analgesics in patients receiving palliative care who are not sufficiently responsive to laxatives1,2 – Brings prompt relief from OIC within 4 hrs1 – Reduces the need for enemas3 – Is generally well tolerated with most treatmentemergent adverse events rated as mild or moderate in severity1 1. 2. 3. Thomas J et al. NEJM 2008;358:2332-43 Holzer Expert Opinion Investig Drugs 2007; 16(2): 181-194 Wyeth Data on File MNTX02/2008 Dosing Schedule The recommended dose of Methylnaltrexone bromide is: • 8 mg (for patients weighing 38-61 kg) • 12 mg (for patients weighing 62-114 kg) • Patients whose weight falls outside of the range should be dosed at 0.15mg/kg • The injection volume for these patients should be calculated: Dose (ml) = patient weight (kg) x 0.0075 Methylnaltrexone should be added to induce prompt bowel movement when response to laxative therapy has been insufficient The usual administration schedule is one single dose every other day. Doses may also be given with longer intervals, as per clinical need. Patients may receive two consecutive doses 24 hours apart, when there has been no response (bowel movement) to the dose on the preceding day Local clinical guidelines may be taken into consideration Methylnaltrexone SmPC July 2008, Wyeth Pharmaceuticals Final comments…… BELIEVE THE PATIENT’S PAIN COMPLAINTS & ESTABLISH REALISTIC PAIN RELIEF GOALS TITRATE OPIOIDS TO PAIN RELIEF PROGRESSION OF THE DISEASE, NOT TOLERANCE, IS THE USUAL CAUSE FOR DOSE INCREASES CONSIDER PALLIATIVE CARE CONSULT FOR PAIN SYMPTOMS