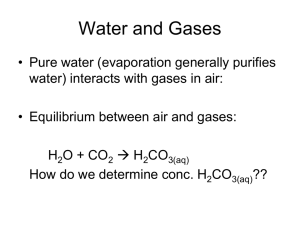
Stoich. Day 7/ Gas Laws Day 1 (Feb. 11-12) Unit 6
advertisement
COPPER LAB PRESENTATIONS! Have one lab member log on to a computer Get out your green packet and yellow Copper Lab Rubric YOU HAVE 5 MINUTES TO POLISH YOUR POWERPOINT! Figure out who is presenting what. Join Google Classroom Turn in Lab •Period 1= ykxs66 •Period 3= e8rw894 •Period 4= ac(zero)uwe •Period 5= rkcuv5r •Period 6= 7n2k8am Find a “partner” lab group • You will be presenting to each other. • Rock paper scissors (winner presents first) • Score them on • Once finished, take a minute and finish up your score • Have other group present • Double check that you have finished your green packet… wait for instructions their rubric on the Peer Score Side Reflection • Write all the lab group members on a piece of paper and score yourself and your lab members out of ten. Please include a brief explanation to defend the scores you gave. 10= super helpful and reliable 7 = was helpful most of the time 5= kinda helpful 3- pretty much dropped the ball 0- might as well have been gone Fold your paper in half so your ratings are hidden and put it in the pink basket • Lab member ___(you)______= XX/10, because… • Lab member ______________= XX/10, because… • Lab member ______________= XX/10, because… • Lab member ______________= XX/10, because… How to turn in your materials Get a gold piece of paper and write your names and beaker code Paperclip in this order 1. Your folded up participation scores 2. Gold piece of paper 3. Yellow Rubric 4. Green Packet Entry Task • Draw a small particle model for each scenario Solid H2O Liquid H2O Gaseous H2O Entry Task • Draw a small particle model for each scenario Solid H2O Liquid H2O Gaseous H2O GAS LAWS Chapter 13 Properties of gases • Gases are made of covalently-bonded non-metals • Ex: CO2, H2, He, O2, NO2 • Gases are easily compressed, fill a container and mix completely with other gases • Gases exert pressure on their surroundings • Ex: air in a balloon, gas in the intestines Atmospheric pressure • Atmospheric pressure = how much pressure atmospheric gases are exerting on us • • Result of gravity pulling air towards earth Measured by a barometer • • • • Bar=pressure Meter=measure At sea level, normal pressure is 760mm Hg We measure in inches of Hg Units used to measure pressure Standard Pressure (at sea level) = • 14.7 lb/in2 or psi, • 101.3 kPa, • 1 kPa = 1000 Pa • 760 mmHg • 1 atm • 1.013 bar Gases & pressure- why care? • Predictor of weather • Shaking a pop can • Scuba diving • Mountain climbing • Space exploration • Your examples?!? Causes of Pressure • Consider this sample of air, the circles represent atoms • What is causing the pressure in this sample? • Is the pressure changing? Why or why not? • What is pressure? Pressure # of particle collisions at a given time. Caused by particles hitting each other AND the walls of the container. Bernoulli’s Principle The flow/speed of molecules changes gas pressure • • As speed increases, pressure decreases As speed decreases, pressure increases Why? • Increasing air speed aligns molecules less collisions, LOWER pressure • Slower air is more chaotic more collisions, HIGH pressure • Everything flows from high to low pressure Bernoulli and lift NO HOMEWORK! Have a great break!