PowerPoint Presentation - Part 2. Water in the Atmosphere
advertisement

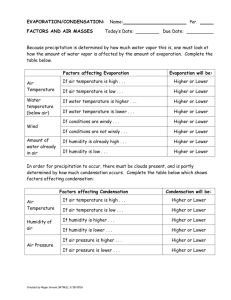
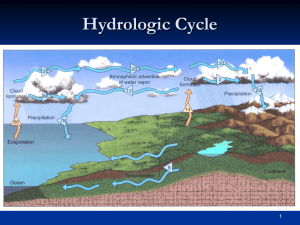
Part 2. Water in the Atmosphere Chapter 5. Atmospheric Moisture The Hydrologic Cycle The Hydrologic Cycle shows how H2O cycles from the surface and subsurface to the atmosphere and back to the surface. Water covers 70% of the Earth’s surface The movement of water vapor molecules exerts vapor pressure Evaporation -- H20 goes from liquid phase to gas phase Condensation -- H20 goes from gas phase to liquid phase Solid surface prevents evaporation Evaporation exceeds Evaporation equals condensation -condensation Saturation vapor pressure Indices of Water Vapor Content Humidity • The amount of water vapor in air – Expressed in many ways Vapor pressure • Pressure exerted on the atmosphere by water vapor – Dependent on temperature and density Saturation Vapor Pressure = maximum water vapor pressure possible (100% humidity) Saturation vapor pressure line: condensation equals evaporation (=100% humidity; saturation) More condensation than evaporation (>100% humidity; supersaturation) More evaporation than condensation (<100% humidity; undersaturation) Measures of Water Vapor Content in the Air • Absolute Humidity • Specific Humidity • Relative Humidity • Mixing Ratio Air Temperature Relative Humidity The relationship between Relative Humidity and Temperature Actual amount of water vapor in air Water vapor content for saturation For same water vapor content: Air at 14°C has relative humidity of 60% Air at 25°C has relative humidity of 30% Dew Point = Temperature above freezing at which saturation occurs (i.e., dew forms) Frost Point = Temperature where saturation occurs below the freezing point (i.e., frost forms) Relative Humidity: 80% 100% 100% When the air temperature drops to the dewpoint, the relative humidity is 100% Conditions that can lead to saturation Addition of water vapor to the air (by evaporation) Mixing cold air with warm, moist air Cooling air to the dew point (by IR radiation) Larger drops have less curvature than smaller ones The greater the curvature of a drop, the greater the rate of evaporation from the drop; very small drops can have supersaturated conditions near them Humidity near droplet surface > 100% Humidity near droplet surface = 100% Small droplets require higher Relative Humidities to remain liquid without completely evaporating Condensation in the atmosphere normally occurs around condensation nuclei. (Water vapor does not condense in pure air.) Condensation nuclei can be dust, ash, spores, soot, salt, etc., also called (hygroscopic nuclei). Dissolved hygroscopic nuclei in water droplets reduce the evaporation rate of the droplets Water droplets in the atmosphere can be supercooled (below 0° C) Deposition (water vapor directly to ice) in the atmosphere occurs around ice nuclei Supercooled water in the atmosphere Atmospheric water does not freeze at 0oC (32oF) Leads to supercooled water At or below -40oC (-40oF) = spontaneous nucleation High Humidities and Human Discomfort Heat index • Combines heat and humidity factors High humidity reduces evaporation • Reduction in the cooling power of perspiration Heat Index Tables Processes for Heating and Cooling Air Diabatic process -- A process that changes the temperature of a gas, liquid or solid through the direct addition or removal of heat energy Adiabatic process -- A process that changes the temperature of a gas, liquid or solid without any addition or removal of heat energy The Second Law of Thermodynamics -- Energy always transfers from areas of higher temperature to areas of lower temperature Dry adiabatic cooling When air rises rapidly without condensation, it cools at the dry adiabatic lapse rate -1oC/100m (-5.5oF/1000ft). When air sinks rapidly without condensation, it warms at the dry adiabatic lapse rate 1oC/100m (5.5oF/1000ft). Saturated (or moist) adiabatic cooling T=8.5°C T=9.0°C T=9.5°C When saturated air rises rapidly, it condenses and cools at the saturated adiabatic lapse rate of about -.5oC/100m (about -3.3oF/1000ft). Air cannot stay saturated when it sinks; it always sinks at the dry adiabatic lapse rate. The Environmental Lapse Rate is the change in air temperature with height as measured by a rising weather balloon The environmental lapse rate changes with the the time of day and variations in wind direction A comparison of adiabatic and environmental cooling rates Air inside rising balloon tries to cool at the adiabatic lapse rate Environmental lapse rate (air temperature outside balloon) Forms of Condensation Dew Liquid condensation on surface objects Frost (white or hoar) Deposition in below-freezing conditions Frozen Dew Dew formation followed by a temperature drop Creates a tight surface bond Radiation Fog • Diabatic chilling of near surface due to radiational cooling • A slight breeze is required Advection Fog Warm, moist air moving over a cooler surface • Diabatic process Upslope Fog Adiabatic process from upslope advection Precipitation Fog Evaporating rain Steam Fog Water evaporated into cold, dry air Radiation fog in the Central Valley Different types of fog found throughout the U.S. Formation and Dissipation of Cloud Droplets Clouds formed through adiabatic cooling of rising air 50 m above the LCL (lifting condensation level) all condensation nuclei are used Additional growth occurs instead of new drop formation