Water - csfcbiology
advertisement

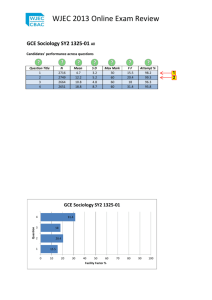
Properties of Water WJEC GCE BIOLOGY Water is important to living organisms because it is a medium for: •Metabolic processes •Transport •Aquatic organisms to live in Properties of Water WJEC GCE BIOLOGY • Water consists of an oxygen atom bound to two hydrogen atoms by two single covalent bonds. – The shared electrons are not shared equally. The oxygen atom pulls the electrons closer to it – This leaves the water molecule with positively and negative charged ends and is therefore called a polar molecule. Water is a Polar Molecule -has oppositely charged ends Water molecules form hydrogen bonds Properties of Water WJEC GCE BIOLOGY slightly positive charge hydrogen bond between (+) and (-) areas of different water molecules slightly negative charge Surface Tension Colour Cohesion and adhesion Properties of Water WJEC GCE BIOLOGY Latent heat State at room temp. Specific heat capacity Density Next Surface Tension • • Properties of Water WJEC GCE BIOLOGY • • • Water has the highest surface tension of any liquid except mercury The surface of water can behave like an elastic sheet, this is due to cohesion between water molecules. Those molecules in the surface are not effected by molecules above them, and therefore ‘pull’ together more strongly, effectively resembling a stretched membrane A habitat can therefore be produced on the surface of the water A pond skater can walk across the surface of the water Back Colour – transmission of light • Water is colourless and is therefore transparent to light • This means that sunlight can reach the cells of water plants so that photosynthesis may occur Reflected by leaves 53% 10% 13% Properties of Water WJEC GCE BIOLOGY Sun light Light absorbed by leaves (typical values): Near IR 15% Red 82% Green 80% Blue 84% 9% 8% 7% 7% 32% Transmitted through leaves Back Cohesion and adhesion • • • Water molecules show a strong attraction to one another due to hydrogen bonding, this is called cohesion. Water molecules can also show attraction to other polar molecules called adhesion The forces of cohesion and adhesion help water travel up the xylem vessels of plants. Properties of Water WJEC GCE BIOLOGY Droplets of water form spheres due to cohesion between water molecules Adhesion and cohesion help water move up xylem Back Latent heat • While changing state, a substance will take in heat energy (solid liquid gas), or expel heat energy (gas liquid solid) without a change in temperature. This is termed Latent Heat. temperature evaporate GAS Properties of Water WJEC GCE BIOLOGY condense • Water has a large latent heat of vaporisation, and therefore absorbs a large amount of heat energy while changing from water to vapour. Lots of energy is needed to break hydrogen bonds • Organisms use this to cool down by the process of sweating. melt solidify time Back State at room temperature • Water is a liquid at room temperature it can therefore be used as a solvent. – This allows chemical reactions to take place in solution – Water can acts as a transport medium carrying dissolved molecules e.g. blood is a transport medium in animals, in plants water transports mineral ions in the xylem. Properties of Water WJEC GCE BIOLOGY • Water is an effective solvent as it can form hydrogen bonds with ions. e.g. Na Cl. – The positive end of the water molecule attracts the negative ion and the negative end of the water molecule attracts the positive ion. – The water molecules surround the ions and they therefore dissolve. Back Specific Heat Capacity • The heat needed to raise the temperature of 1kg of water by 1°C is termed the Specific Heat Capacity. • Water has a large specific heat capacity, and therefore can absorb large amounts of heat energy before its temperature raises a significant amount. Again it takes a lot of energy to break hydrogen bonds for molecules to move around. Properties of Water WJEC GCE BIOLOGY thermometer 1kg of water • This prevents large fluctuations in the temperature of water in the environment e.g ensures a stable environment for aquatic organisms Back Density of water • Unlike other substances, water expands as it freezes. It has it’s H H maximum density at 40C O Ice Water H H H H H H O O O H H O H H O H Properties of Water WJEC GCE BIOLOGY Water molecules are able to approach one another quite closely O H H H O O Water molecules in ice form a rigid structure so that there is more space between them, this is seen as expansion as a whole H H H H O O H H • As water expands when it freezes, its density (mass per unit volume) will decrease. • This means that ice is less dense than water, and will therefore float on top of it. • In aquatic environments, ice forms an insulatory layer and prevents the entire water column from freezing Back Properties of Water WJEC GCE BIOLOGY Match the property of water with the corresponding significance for life and correctly fill in the table START Property of water Ice is less dense than water The high surface tension of water means that it can form a habitat on the surface of the water High surface tension Ice forms an insulating layer over water Strong cohesive properties and high tensile strength Colourless with a high transmission Properties of Water Liquid at room temperature WJEC GCE BIOLOGY Significance for life Answers In order to evaporate it must absorb a large amount of energy (high latent heat of vaporisation) Water can absorb a lot of energy for only a small rise in temperature (high specific heat capacity) Can be used for cooling organisms by evaporation of for example sweat Conditions are stable in cells and aquatic environments Can be used for transport and a medium for reactions Light can pass through cells for photosynthesis Water can be pulled through plants in a column as the water molecules are held together by H-bonds Property of water Ice is less dense than water Ice forms an insulating layer over water High surface tension The high surface tension of water means that it can form a habitat on the surface of the water Properties of Water Strong cohesive properties and high tensile strength WJEC GCE BIOLOGY Significance for life Water can be pulled through plants in a column as the water molecules are held together by H-bonds Colourless with a high transmission Light can pass through cells for photosynthesis Liquid at room temperature Can be used for transport and a medium for reactions In order to evaporate it must absorb a large amount of energy (high latent heat of vaporisation) Can be used for cooling organisms by evaporation of for example sweat Water can absorb a lot of energy for only a small rise in temperature (high specific heat capacity) Conditions are stable in cells and aquatic environments Next