Blood clotting factors (proteins)
advertisement

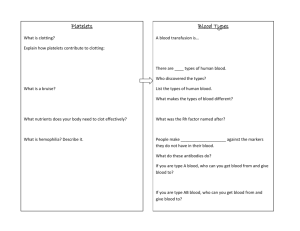
Blood Main features • Blood cells and blood plasma • Plazma proteins cause blood clotting and form thrombs. • Serum does not contain white or red blood cells nor a clotting factor; it is the blood plasma with the fibrinogens removed. • Blood cells are: erythrocytes, leukocytes and platelets http://en.wikipedia.org/wiki/Blood Formed elements Blood plasma Blood plasma - 55% of blood volume. 90% - 92% water, 7%-9% proteins, 1% inorganic elements, glucose, hormons, vitamins, a.o. Tissue liquid – similar concentration of electrolyte, smaller concentration of proteīns a.o. organic substances. Border: basal membrane and wall of blood vessels with Continuous or Fenestrated endothelium. Ions Ions Concentration (mmol.l−1) Calcium Chlorine Potassium Sodium 2.1–2.8 95–105 3.6–5.1 135–145 Proteins Protein Albumin Koncentrācija 3.5-5.0 g/dl % Function 55% maintains colloid osmotic pressure; create oncotic pressure and transport insoluble molecules Globulins 2.0-2.5 g/dl 38% α and β globulins transport metals, vitamins, a.o., γ tubulin (antibodies) involved in immune response Fibrinogen 0.2-0.45 g/dl 7% Blood clotting Regulatory proteins <1% Regulate gene expression a.o. cell functions Blood clotting factors (proteins) <1% Transform fibrinogen into fibrine Complement Cascade of protein activation, to attach them to antigens and cause destruction of foreign cells in human body. http://highered.mcgrawhill.com/sites/0072507470/student_view0/chapter22/animation__activation_of_complement.html Blood clotting http://www.youtube.com/watch?v=xNZEERMSeyM Erytrocytes http://www.pbrc.hawaii.edu/~kunkel/gallery. Erythrocytes (red), leukocytes (green) un platelets (yellow). (SEM x 9,900). Erythrocytes can move through capillaries with diameter smaller than diameter of erythrocyte. carbohydrates carbohydrates Extracellular matrix Protein 3 Glycophorin ß -Spektrin Actin Protein 4,1 Cholesterol Phosfolipids Glicolipids -Spectrin -Ankirin Protein 4,1 Protein 4,2 Tropomiosin Plsmatic membrane of an erythrocyte Band 3 protein - Cl-/HCO3 antiporter Glikophorin – negative charge on membrane surface • Erythrocytes change their shape during the movement. • Colour depends on concentration of CO2 • Hemoglobin - 4 polipeptide chains. Functional group – heme, contains Fe atoms. • Content: 65% water, 1% organelles, 9% hemoglobine. Fe http://en.wikipedia.org/wiki/File:Erytrocyte_deoxy_to_oxy_v0.7.gif Human blood group system • Glycolipids A and B (aglutinogens) • 0-I group, A -II groupa, B-III groupa, AB-IV groupa. • Plazma contain proteins – aglutins. Can be attached to aglutinogens. Sickle cell anemia Rhesus factor • 50 aglutinogens • most important C,D,E aglutinogens • Cause hemolysis of fetus if in the blood of “Rh-” mother antibodies against Rh becomes synthesized after first labour. Classification of leukocytes • Granulocytes – contain specifc and unspecific granules • Agranulocytes – do not contain granules Cell structure after staining can be: eosinophilic (acidophilic), basophilic (stain with methilene blue) and azurophilic (purple), light purple (weak staining with eosin un methilene blue, specific granules) Leukocyte differencial analysis • • • • • • Number of leukocytes in addults: Neutrophilic granulocytes - 2.0–7.0×109/l (40–80%) Limphocytes - 1.0–3.0×109/l (20–40%) Monocytes - 0.2–1.0×109/l (2–10%) Eosinophilic granulocytes - 0.02–0.5×109/l (1–6%) Basophilic granuloccytes- 0.02–0.1×109/l (< 1–2%) • Numbers can differ in different populations and in different laborotories. • Immature granulocyte (IG) • Immature granulocytes (IGs) encompass immature cells of granulocytic lineages, including metamyelocytes, myelocytes, and promyelocytes, which are easily recognized morphologically and are reported by automated analyzer as IG altogether. IG normally absent from peripheral blood. • Increased IG occurs accompanied by an increase in neutrophils in the following conditions:[2] • Bacterial infections • Acute inflammatory diseases • Cancer (particularly with marrow metastasis) • Tissue necrosis • Acute transplant rejection • Surgical and orthopedic trauma • Myeloproliferative diseases • Steroid use • Pregnancy (mainly during the third trimester) • Neutrophil granulocytes — May indicate bacterial infection. May also be raised in acute viral infections. Because of the segmented appearance of the nucleus, neutrophils are sometimes referred to as "segs." The nucleus of less mature neutrophils is not segmented, but has a band or rod-like shape. Less mature neutrophils — those that have recently been released from the bone marrow into the bloodstream — are known as "bands" or "stabs". Stab is a German term for rod.[3] • Lymphocytes — Higher with some viral infections such as glandular fever and. Also raised in chronic lymphocytic leukemia (CLL). Can be decreased by HIV infection. In adults, lymphocytes are the second most common WBC type after neutrophils. In young children under age 8, lymphocytes are more common than neutrophils.[3] • Monocytes — May be raised in bacterial infection, tuberculosis, malaria, Rocky Mountain spotted fever, monocytic leukemia, chronic ulcerative colitis and regional enteritis [3] • Eosinophil granulocytes — Increased in parasitic infections, asthma, or allergic reaction. • Basophil granulocytes — May be increased in bone marrow related conditions such as leukemia or lymphoma.[3] Monocytes un macrophages Cell Name Location Adipose tissue macrophages Adipose tissue Monocyte Bone Marrow/Blood Kupffer cell Liver Sinus histiocytes Lymph node Alveolar macrophage(dust cell) Pulmonary alveolus of Lungs Tissue macrophage (Histiocyte) leading to Giant cells Connective Tissues Langerhans cell Skin and Mucosa Microglia Central Nervous System Hofbauer cell Placenta Intraglomerular mesangial cell Kidney Osteoclasts Bone Epithelioid cells Granulomas Red Pulp Macrophage (Sinusoidal lining cells) Red pulp of Spleen Peritoneal macrophages Peritoneal cavity • Monocytes and macrophages are phagocytes.[3] Macrophages function in both non-specific defense (innate immunity) as well as help initiate specific defense mechanisms (adaptive immunity) of vertebrate animals. • Macrophages have the unique ability to metabolize one amino acid, arginine, to either a "killer" molecule (Nitric Oxide) or a "repair" molecule (Ornithine). • Macrophages predominantly expressing the killer or repair phenotype are now mainly called M1 or M2 macrophages because these 2 types of macrophages also stimulate T cell responses that further activate the killer macrophages or T cell phenotype (Th1), or stimulate antibody production (Th2) Monocytes • Large number of lysosomes. Makrophages • Beta glikans and other signalling molecules activate macrophages Macrophages 1. Erithrocytes; 2. – cytoplasm; 3. - nucleus, 4. – bacteria containing endosomes; 5. - pseudopodia. Scale bar: 10 μm. Specific ekxpresion of proteins CD14, CD40, CD11b, CD64, F4/80 (pele)/EMR1 (cilvēks), lysozyme M, MAC-1/MAC-3 and CD68 by flow cytometry or immunohistochemical staining.[4] Macrophages www.beta-glucan-13d.com/ http://www.life.umd.edu/classroom/bsci422/mosser/macrophage.jpg Macrophages and cytoskeleton • http://www.svi.nl/products/software/fluvr/ • When a monocyte enters damaged tissue through the endothelium of a blood vessel, a process known as the leukocyte extravasation, it undergoes a series of changes to become a macrophage. • Monocytes are attracted to a damaged site by chemical substances through chemotaxis, triggered by a range of stimuli including damaged cells, pathogens and cytokines released by macrophages already at the site. • At some sites such as the testis, macrophages have been shown to populate the organ through proliferation. • Unlike short-lived neutrophils, macrophages survive longer in the body up to a maximum of several months. http://en.wikipedia.org/wiki/Macrophage • • • • Role in adaptive immunity Macrophages are versatile cells that play many roles. As scavengers, they rid the body of worn-out cells and other debris. Along with dendritic cells, they are foremost among the cells that "present" antigen, a crucial role in initiating an immune response. As secretory cells, monocytes and macrophages are vital to the regulation of immune responses and the development of inflammation; they produce a wide array of powerful chemical substances (monokines) including enzymes, complement proteins, and regulatory factors such as interleukin-1. At the same time, they carry receptors for lymphokines that allow them to be "activated" into single-minded pursuit of microbes and tumour cells. http://en.wikipedia.org/wiki/Macrophage • After digesting a pathogen, a macrophage will present the antigen (a molecule, most often a protein found on the surface of the pathogen and used by the immune system for identification) of the pathogen to the corresponding helper T cell. • The presentation is done by integrating it into the cell membrane and displaying it attached to an MHC class II molecule, indicating to other white blood cells that the macrophage is not a pathogen, despite having antigens on its surface. • Eventually, the antigen presentation results in the production of antibodies that attach to the antigens of pathogens, making them easier for macrophages to adhere to with their cell membrane and phagocytose. In some cases, pathogens are very resistant to adhesion by the macrophages. http://en.wikipedia.org/wiki/Macrophage Antigen presentation • The antigen presentation on the surface of infected macrophages (in the context of MHC class II) in a lymph node stimulates TH1 (type 1 helper T cells) to proliferate (mainly due to IL-12 secretion from the macrophage). • When a B-cell in the lymph node recognizes the same unprocessed surface antigen on the bacterium with its surface bound antibody, the antigen is endocytosed and processed. • The processed antigen is then presented in MHCII on the surface of the B-cell. • T cells that express the T cell receptor which recognizes the antigen-MHCII complex (with co-stimulatory factors- CD40 and CD40L) cause the B-cell to produce antibodies that help opsonisation of the antigen so that the bacteria can be better cleared by phagocytes. • Macrophages provide yet another line of defense against tumor cells and somatic cells infected with fungus or parasites. • Once a T cell has recognized its particular antigen on the surface of an aberrant cell, the T cell becomes an activated effector cell, producing chemical mediators known as lymphokines that stimulate macrophages into a more aggressive form. http://en.wikipedia.org/wiki/Macrophage • In spite of a spectrum of ways to activate macrophages, there are two main groups designated M1 and M2. • M1 macrophages, as mentioned earlier (previously referred to as classically or alternatively activated macrophages),[7] M1 "killer" macrophages are activated by LPS and IFN-gamma, and secrete high levels of IL-12 and low levels of IL-10. • In contrast, the M2 "repair" designation broadly refers to macrophages that function in constructive processes like wound healing and tissue repair, and those that turn off damaging immune system activation by producing anti-inflammatory cytokines like IL-10. • M2 is the phenotype of resident tissue macrophages, and can be further elevated by IL-4. • M2 macrophages produce high levels of IL-10, TGF-beta and low levels of IL-12. Tumor-associated macrophages are mainly of the M2 phenotype, and seem to actively promote tumor growth.[8] http://en.wikipedia.org/wiki/Macrophage Role in muscle regeneration • The first step to understanding the importance of macrophages in muscle repair, growth, and regeneration is that there are two “waves” of macrophages with the onset of damageable muscle use – subpopulations that do and do not directly have an influence on repairing muscle. • The initial wave is a phagocytic population that comes along during periods of increased muscle use that are sufficient to cause muscle membrane lysis and membrane inflammation, which can enter and degrade the contents of injured muscle fibers.[9][10][11] • These early-invading, phagocytic macrophages reach their highest concentration about 24 hours following the onset of some form of muscle cell injury or reloading.[12] • Their concentration rapidly declines after 48 hours.[10] • The second group is the non-phagocytic types that are distributed near regenerative fibers. These peak between two and four days and remain elevated for several days during the hopeful muscle rebuilding.[10] • The first subpopulation has no direct benefit to repairing muscle, while the second non-phagocytic group does. http://en.wikipedia.org/wiki/Macrophage • It is thought that macrophages release soluble substances that influence the proliferation, differentiation, growth, repair, and regeneration of muscle, but at this time the factor that is produced to mediate these effects is unknown.[12] • It is known that macrophages' involvement in promoting tissue repair is not muscle specific; they accumulate in numerous tissues during the healing process phase following injury.[13] • A study conducted in 2006 showcased macrophage influences on muscle repair of soleus muscle on mice.[14] • The first procedural step was to make sure macrophages are present in the muscle after onset of muscle injury, and then decrease their presence to see what effects were had on the muscle. • By using anti-F4/80 to bind to macrophages and render them useless, it was seen that when the second wave of macrophages were depleted, there were many more lesions in the muscle cell membrane between the second and fourth day – showing muscle damage when repairing is supposed to occur. • After testing for membrane lesions in both the total amount of muscle fibers present, it was noticed that most of the damage occurred in muscle cells that did not have the second subpopulation of macrophages present. Macrophages depletion prevents muscle membrane repair. http://en.wikipedia.org/wiki/Macrophage • When examining muscle regeneration, a significant reduction was found in the amount of myonuclei. • Depletion of macrophages was found to cause, between the second and fourth day of repair, much less muscle regeneration compared to muscle with macrophage population.[14] • Macrophages promote muscle regeneration between the second and fourth day. • To determine the influence of macrophages in muscle growth, muscle crosssectional area in macrophage-depleted muscle area was measured against two muscle sets: muscle that was injured and had macrophage presence and muscle that was not injured and had macrophage presence. • The macrophage-depleted muscle showed less growth after four days, and injured muscle with macrophages nearly grew back to the level of uninjured muscle.[14] http://en.wikipedia.org/wiki/Macrophage • Role in wound healing • Macrophages replace Polymorphonuclear neutrophils as the predominant cells in the wound by two days after injury.[16] • Attracted to the wound site by growth factors released by platelets and other cells, monocytes from the bloodstream enter the area through blood vessel walls.[17] • Numbers of monocytes in the wound peak one to one and a half days after the injury occurs. Once they are in the wound site, monocytes mature into macrophages. The spleen contains half the body's monocytes in reserve ready to be deployed to injured tissue.[18][19] • The macrophage's main role is to phagocytize bacteria and damaged tissue,[15] and they also debride damaged tissue by releasing proteases.[20] • Macrophages secrete a number of factors such as growth factors and other cytokines, especially during the third and fourth post-wounding days. These factors attract cells involved in the proliferation stage of healing to the area.[21] • Macrophages may also restrain the contraction phase.[22] Macrophages are stimulated by the low oxygen content of their surroundings to produce factors that induce and speed angiogenesis [23] and they also stimulate cells that reepithelialize the wound, create granulation tissue, and lay down a new extracellular matrix.[24][25] http://en.wikipedia.org/wiki/Macrophage Neutrophilic granulocytes 11.11.2013 Eozinophilic granulocytes • Eosinophils also have lobed nuclei (two to four lobes). The number of granules in an eosinophil can vary because they have a tendency to degranulate while in the blood stream.[15] • Eosinophils play a crucial part in the killing of parasites (e.g., enteric nematodes) because their granules contain a unique, toxic basic protein and cationic protein (e.g., cathepsin[12]);[16] receptors that bind to IgE are used to help with this task.[17] • These cells also have a limited ability to participate in phagocytosis,[18] they are professional antigen-presenting cells, they regulate other immune cell functions (e.g., CD4+ T cell, dendritic cell, B cell, mast cell, neutrophil, and basophil functions),[19] they are involved in the destruction of tumor cells,[15] and they promote the repair of damaged tissue.[20] A polypeptide called interleukin-5 interacts with eosinophils and causes them to grow and differentiate; this polypeptide is produced by basophils.[16] http://en.wikipedia.org/wiki/Granulocyte Basophylic granulcytes • Basophils are one of the least abundant cells in bone marrow and blood (occurring at less than two percent of all cells). Like neutrophils and eosinophils, they have lobed nuclei; however, they have only two lobes, and the chromatin filaments that connect them are not very visible. • Basophils have receptors that can bind to IgE, IgG, complement, and histamine. The cytoplasm of basophils contains a varied amount of granules; these granules are usually numerous enough to partially conceal the nucleus. Granule contents of basophils are abundant with histamine, heparin, chondroitin sulfate, peroxidase, platelet-activating factor, and other substances. http://en.wikipedia.org/wiki/Granulocyte • When an infection occurs, mature basophils will be released from the bone marrow and travel to the site of infection.[21] When basophils are injured, they will release histamine, which contributes to the inflammatory response that helps fight invading organisms. • Histamine causes dilation and increased permeability of capillaries close to the basophil. • Injured basophils and other leukocytes will release another substance called prostaglandins that contributes to an increased blood flow to the site of infection. Both of these mechanisms allow blood-clotting elements to be delivered to the infected area (this begins the recovery process and blocks the travel of microbes to other parts of the body). • Increased permeability of the inflamed tissue also allows for more phagocyte migration to the site of infection so that they can consume microbes.[18] http://en.wikipedia.org/wiki/Granulocyte Heparinocytes • Mast are now considered to be part of the immune system. • Mast cells are very similar to basophil granulocytes (a class of white blood cells) in blood. • Both are granulated cells that contain histamine and heparin, an anticoagulant. Both cells also release histamine upon binding to immunoglobulin E.[3] • These similarities have led many to speculate that mast cells are basophils that have "homed in" on tissues. Furthermore they share a common precursor in bone marrow expressing the CD34 molecule. • Basophils leave the bone marrow already mature, whereas the mast cell circulates in an immature form, only maturing once in a tissue site. The site an immature mast cell settles in probably determines its precise characteristics.[2] • The first in vitro differentiation and growth of a pure population of mouse mast cells has been carried out using conditioned medium derived from concanavalin A-stimulated splenocytes.[6] • Later, it was discovered that T cell-derived interleukin 3 was the component present in the conditioned media that was required for mast cell differentiation and growth.[7] http://en.wikipedia.org/wiki/Mast_cell http://en.wikipedia.org/wiki/Mast_cell Megakariocytes produce thrombocytes • Megakariocyte in red bone marrow. (left side) • Megakarioc form thrombocytes. Gimsza stain. (right side) Thrombocytes lack major organelles. Thrombocytes of nonmammalian vertebrates have a nucleus and resemble B lymphocytes. Mammalian thrombocytes are anucleated cells called platelets which additionally aggregate in response to ADP, serotonin, and adrenaline. www.ipfdd.de/research/ res16/a18/a18.html Trombocīti veido agregātus. www.explorepub.com/articles/darkfield_charts/ fungus5.html • If the number of platelets is too low, excessive bleeding can occur. • However, if the number of platelets is too high, blood clots can form (thrombosis), which may obstruct blood vessels and result in such events as a stroke, myocardial infarction, pulmonary embolism or the blockage of blood vessels to other parts of the body, such as the extremities of the arms or legs. • An abnormality or disease of the platelets is called a thrombocytopathy,[2] which could be either a low number of platelets (thrombocytopenia), a decrease in function of platelets (thrombasthenia), or an increase in the number of platelets (thrombocytosis). • There are disorders that reduce the number of platelets, such as heparin-induced thrombocytopenia (HIT) or thrombotic thrombocytopenic purpura (TTP) that typically cause thromboses, or clots, instead of bleeding. http://en.wikipedia.org/wiki/Platelets • Platelets release a multitude of growth factors including plateletderived growth factor (PDGF), a potent chemotactic agent, and TGF beta, which stimulates the deposition of extracellular matrix. • Both of these growth factors have been shown to play a significant role in the repair and regeneration of connective tissues. • Other healing-associated growth factors produced by platelets include basic fibroblast growth factor, insulin-like growth factor 1, plateletderived epidermal growth factor, and vascular endothelial growth factor. • Local application of these factors in increased concentrations through Platelet-rich plasma (PRP) has been used as an adjunct to wound healing for several decades. http://en.wikipedia.org/wiki/Platelets • The physiological range for platelets is (150 – 400) × 103 per mm3. • Platelets are produced in blood cell formation (thrombopoiesis) in bone marrow, by budding off from megakaryocytes. • Megakaryocyte and platelet production is regulated by thrombopoietin, a hormone usually produced by the liver and kidneys. • Each megakaryocyte produces between 5,000 and 10,000 platelets. • Around 1011 platelets are produced each day by an average healthy adult. • Reserve platelets are stored in the spleen, and are released when needed by sympathetically induced splenic contraction. • The lifespan of circulating platelets is 5 to 9 days. • Old platelets are destroyed by phagocytosis in the spleen and by Kupffer cells in the liver. http://en.wikipedia.org/wiki/Platelets • The inner surface of blood vessels is lined with a thin layer of endothelial cells that, in normal hemostasis, acts to inhibit platelet activation by producing nitric oxide, endothelial-ADPase, and PGI2. Endothelial-ADPase clears away the platelet activator, ADP. • Endothelial cells produce a protein called von Willebrand factor (vWF), a cell adhesion ligand, which helps endothelial cells adhere to collagen in the basement membrane. • Under physiological conditions, collagen is not exposed to the bloodstream. • vWF is secreted constitutively into the plasma by the endothelial cells, and is stored in granules within the endothelial cell and in platelets. http://en.wikipedia.org/wiki/Platelets • When the endothelial layer is injured, collagen, vWF and tissue factor from the subendothelium is exposed to the bloodstream. • When the platelets contact collagen or vWF, they are activated (e.g. to clump together). • They are also activated by thrombin (formed with the help of tissue factor). • They can also be activated by a negatively charged surface, such as glass. Non-physiological flow conditions (especially high values of shear stress) caused by arterial stenosis or artificial devices (Mechanical Heart Valves, blood pumps etc.) can also lead to platelet activation.[10] • Platelet activation further results in the scramblase-mediated transport of negatively charged phospholipids to the platelet surface. • These phospholipids provide a catalytic surface (with the charge provided by phosphatidylserine and phosphatidylethanolamine) for the tenase and prothrombinase complexes. • Calcium ions are essential for binding of these coagulation factors. http://en.wikipedia.org/wiki/Platelets Granule secretion • Platelets contain alpha and dense granules. • Activated platelets excrete the contents of these granules into their canalicular systems and into surrounding blood. • There are three types of granules: 1. dense (or delta) granules (containing ADP or ATP, calcium, and serotonin) 2. lambda granules – similar to lysosomes and contain several hydrolytic enzymes. 3. Alpha granules (containing P-selectin, platelet factor 4, transforming growth factor-β1, platelet-derived growth factor, fibronectin, B-thromboglobulin, vWF, fibrinogen, and coagulation factors V and XIII). • Thromboxane A2 synthesis[edit] • Platelet activation initiates the arachidonic acid pathway to produce TXA2. TXA2 is involved in activating other platelets and its formation is inhibited by COX inhibitors, such as aspirin. http://en.wikipedia.org/wiki/Platelets • Platelets aggregate, or clump together, using fibrinogen and von Willebrand factor (vWF) as a connecting agent. • The most abundant platelet aggregation receptor is glycoprotein IIb/IIIa (gpIIb/IIIa); this is a calcium-dependent receptor for fibrinogen, fibronectin, vitronectin, thrombospondin, and vWF. Other receptors include GPIb-V-IX complex (vWF) and GPVI (collagen). • Activated platelets will adhere, via glycoprotein (GP) Ia, to the collagen that is exposed by endothelial damage. • Aggregation and adhesion act together to form the platelet plug. • Myosin and actin filaments in platelets are stimulated to contract during aggregation, further reinforcing the plug. • Platelet aggregation is stimulated by ADP, thromboxane, and α2 receptoractivation, but inhibited by other inflammatory products like PGI2 and PGD2. • Platelet aggregation is enhanced by exogenous administration of anabolic steroids. http://en.wikipedia.org/wiki/Platelets • Wound repair • Main article: Wound repair • The blood clot is only a temporary solution to stop bleeding; vessel repair is therefore needed. The aggregated platelets help this process by secreting chemicals that promote the invasion of fibroblasts from surrounding connective tissue into the wounded area to completely heal the wound or form a scar. The obstructing clot is slowly dissolved by the fibrinolytic enzyme, plasmin, and the platelets are cleared by phagocytosis. • ADP (purinergic/P2) receptors • Human platelets have three types of P2 receptors: P2X(1), P2Y(1) and P2Y(12). Although abnormalities in all three genes have been documented, but clinical correlation is available only for P2Y(12).[11] Patients with P2Y(12) defects have a mild to moderate bleeding diathesis, characterized by mucocutaneous bleeding and excessive post-surgical and post-traumatic blood loss. A defects in P2Y(12) should be suspected when ADP, even at concentrations ≥10 micro molar, is unable to induce full, irreversible platelet aggregation. Confirmation of the diagnosis is with tests that evaluate the degree of inhibition of adenylyl cyclase by ADP. • Cytokine signaling • In addition to being the chief cellular effector of hemostasis, platelets are rapidly deployed to sites of injury or infection, and potentially modulate inflammatory processes by interacting with leukocytes and by secreting cytokines, chemokines, and other inflammatory mediators.[13][14][15][16] Platelets also secrete plateletderived growth factor (PDGF). Limphocytes http://www.vet.uga.edu/IVCVM/1998/latimer1/latimer1.htm Limphocytes http://www.vet.uga.edu/IVCVM/1998/latimer1/latimer1.htm Limphocytes http://www.lab.anhb.uwa.edu.au/mb140/CorePages/Blood/Blood.htm#Leukocytes • Lymphocytes can be divided into large lymphocytes and small lymphocytes. Large granular lymphocytes include natural killer cells (NK cells). Small lymphocytes consist of T cells and B cells. http://en.wikipedia.org/wiki/Lymphocyte T and B cells • T cells (thymus cells) and B cells (bursa-derived cells[2]) are the major cellular components of the adaptive immune response. • T cells are involved in cell-mediated immunity, whereas B cells are primarily responsible for humoral immunity (relating to antibodies). • The function of T cells and B cells is to recognize specific “non-self” antigens, during a process known as antigen presentation. Once they have identified an invader, the cells generate specific responses that are tailored to maximally eliminate specific pathogens or pathogen-infected cells. • B cells respond to pathogens by producing large quantities of antibodies which then neutralize foreign objects like bacteria and viruses. • In response to pathogens some T cells, called T helper cells, produce cytokines that direct the immune response, while other T cells, called cytotoxic T cells, produce toxic granules that contain powerful enzymes which induce the death of pathogen-infected cells. • Following activation, B cells and T cells leave a lasting legacy of the antigens they have encountered, in the form of memory cells. • Throughout the lifetime of an animal these memory cells will “remember” each specific pathogen encountered, and are able to mount a strong and rapid response if the pathogen is detected again. Natural killer cell • NK cells are a part of the innate immune system and play a major role in defending the host from both tumors and virally infected cells. • NK cells distinguish infected cells and tumors from normal and uninfected cells by recognizing changes of a surface molecule called MHC (major histocompatibility complex) class I. • NK cells are activated in response to a family of cytokines called interferons. • Activated NK cells release cytotoxic (cell-killing) granules which then destroy the altered cells.[3] • They were named "natural killer cells" because of the initial notion that they do not require prior activation in order to kill cells which are missing MHC class I. • Microscopically, in a Wright's stained peripheral blood smear, a normal lymphocyte has a large, dark-staining nucleus with little to no eosinophilic cytoplasm. • The coarse, dense nucleus of a lymphocyte is approximately the size of a red blood cell (about 7 micrometres in diameter).[4] • Some lymphocytes show a clear perinuclear zone (or halo) around the nucleus or could exhibit a small clear zone to one side of the nucleus. • Polyribosomes are a prominent feature in the lymphocytes and can be viewed with an electron microscope.[4] The ribosomes are involved in protein synthesis, allowing the generation of large quantities of cytokines and immunoglobulins by these cells. • It is impossible to distinguish between T cells and B cells in a peripheral blood smear.[4] • Normally, flow cytometry testing is used for specific lymphocyte population counts. • This can be used to specifically determine the percentage of lymphocytes that contain a particular combination of specific cell surface proteins, such as immunoglobulins or cluster of differentiation (CD) markers or that produce particular proteins (for example, cytokines using intracellular cytokine staining (ICCS)). Typical recognition markers for lymphocytes[6] CLASS FUNCTION PROPO RTION PHENOTYPIC MARKER(S) NK cells Lysis of virally infected cells and tumour cells 7% (213%) CD16 CD56 but not CD3 Helper T cells Release cytokines and growth factors that regulate other immune cells 46% (28- TCRαβ, CD3 and 59%) CD4 Cytotoxic T cells Lysis of virally infected cells, tumour cells and allografts 19% (13- TCRαβ, CD3 and 32%) CD8 γδ T cells Immunoregulation and cytotoxicity 5% (2%TCRγδ and CD3 8%) B cells Secretion of antibodies 23% (18- MHC class II, CD19 47%) and CD21