The RAVEL Trial - Clinical Trial Results
advertisement

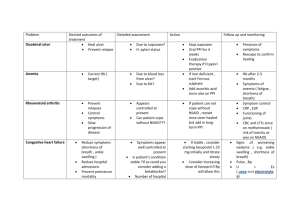
ALLHAT Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial JAMA 2002;288:2981-2997 ALLHAT 42,418 patients with hypertension SBP >140mmHg and/or DBP >90 mmHg OR Took medication for hypertension and had at least one additional risk factor for CHD Age >55 years NHLBI funded trial Calcium Channel Blocker Amlodipine 2.5-10 mg/day (n=9,048) Diuretic Chlorthalidone 12-25 mg/day (n=15,255) ACE Inhibitor Lisinopril 10-40 mg/day (n=9,054) Alpha Blocker Doxazosin* 2-8 mg/day (n=9,061) Endpoints: Primary – Fatal coronary heart disease and nonfatal MI Secondary – All-cause mortality, stroke, and major cardiovascular disease events (CHF, coronary revascularization, angina, and peripheral artery disease) Mean follow-up 4.9 years www. Clinical trial results.org * Discontinued prior to study completion JAMA 2002;288:2981-2997 ALLHAT: Primary Endpoint* 15% Chlorthalidone vs Amlodipine Primary Endpoint RR = 0.98 p = 0.65 11.5% 15% 11.5% 11.3% 10% 10% 5% 5% 0% 0% Chlorthalidone Amlodipine www. Clinical trial results.org Chlorthalidone vs Lisinopril Primary Endpoint RR = 0.99 p = 0.81 Chlorthalidone * Primary Endpoint = Fatal CHD or nonfatal MI 11.4% Lisinopril JAMA 2002;288:2981-2997 ALLHAT: Secondary Endpoints 20% Chlorthalidone vs Amlodipine All Cause Mortality RR = 0.96 15% p = 0.20 17.3% Heart Failure RR = 1.38 p < 0.001 16.8% 10.2% 15% 10% 7.7% 10% 5% 5% 0% 0% Chlorthalidone Amlodipine www. Clinical trial results.org Chlorthalidone Amlodipine JAMA 2002;288:2981-2997 ALLHAT: Secondary Endpoints Chlorthalidone vs Lisinopril 20% All Cause Mortality RR = 1.00 p = 0.90 17.3% 15% Heart Failure RR = 1.19 p < 0.001 10% Stroke RR = 1.15 p = 0.02 17.2% 8% 15% 10% 8.7% 7.7% 6.3% 6% 5.6% 10% 4% 5% 5% 2% 0% Chlorthalidone Lisinopril www. Clinical trial results.org 0% Chlorthalidone Lisinopril 0% Chlorthalidone Lisinopril JAMA 2002;288:2981-2997 ALLHAT: Summary Prespecified primary endpoint of fatal CHD or nonfatal MI did not differ between initial use of the diuretic chlorthalidone vs initial use of the ACE inhibitor lisinopril or the calcium antagonist amlodipine for the treatment of hypertension – Secondary outcome of heart failure was lower among patients treated with chlorthalidone vs lisinopril or amlodipine – Each of the 3 drugs reduced blood pressure from baseline, although chlorthalidone use was associated with larger SBP reductions vs lisinopril or amlodipine – Increased risk of heart failure in lisinopril arm unexpected and in contrast to the benefits of ACE inhibitors observed in other trials for the treatment of heart failure such as SOLVD www. Clinical trial results.org ALLHAT: Limitations Diabetic risk – Important side effect in the chlorthalidone arm was higher fasting glucose levels vs lisinopril or amlodipine arms in all patients and in non-diabetics – Impact of chlorthalidone on diabetes and cardiovascular disease may not be fully manifested in the relatively short follow-up period of 4 years – ACE inhibitors have previously been associated with a reduction in the development of diabetes and the progression of diabetic nephropathy Add-on therapy – ACE inhibitor arm potentially at a disadvantage since the first add-on therapy specified by the trial treatment algorithm for this arm was a beta-blocker rather than a diuretic or calcium channel blocker, both of which are more commonly used in clinical practice Large crossover rate by 4 year follow-up www. Clinical trial results.org