Chapter 7
advertisement

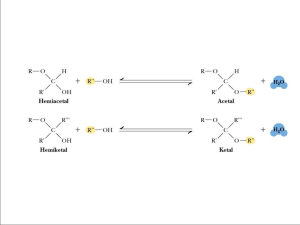
Reginald H. Garrett Charles M. Grisham www.cengage.com/chemistry/garrett Chapter 7 Carbohydrates and the Glycoconjugates of Cell Surfaces Reginald Garrett & Charles Grisham • University of Virginia Outline • How are carbohydrates named? • What is the structure and chemistry of monosaccharides? • What is the structure and chemistry of oligosaccharides? • What is the structure and chemistry of polysaccharides? • What are glycoproteins, and how do they function in cells? • How do proteoglycans modulate processes in cells and organisms? • Do carbohydrates provide a structural code? 7.1 How Are Carbohydrates Named? Carbohydrates are hydrates of carbon with the general formula (CH2O)n Carbohydrates are classified in three groups: • Monosaccharides (simple sugars) - cannot be broken down into simpler sugars under mild conditions • Oligosaccharides (oligo = "a few“) - usually 2 to 10 simple sugar residues • Polysaccharides - polymers of the simple sugars 7.2 What Is the Structure and Chemistry of Monsaccharides? An organic chemistry review • Aldoses and ketoses contain aldehyde and ketone functions, respectively • Triose, tetrose, etc. denotes number of carbons • Aldoses with 3 or more carbon atoms and ketoses with 4 or more carbon atoms are chiral • Review Fischer projections and D,L system 7.2 What Is the Structure and Chemistry of Monsaccharides? Structure of a simple aldose and a simple ketose. The Aldoses The structure and stereochemical relationships of D-aldoses with three to six carbons. Know the ones in the boxes The Ketoses The structure and stereochemical relationships of D-ketoses with three to six carbons. Know the ones in the boxes. Cyclic monsaccharide structures and anomeric forms • • • • • Glucose (an aldose) can cyclize to form a cyclic hemiacetal Fructose (a ketose) can cyclize to form a cyclic hemiketal When hemiacetals and hemiketals are formed, the carbonyl carbon atom becomes an asymmetric center Isomers of monosaccharides that differ only in their configuration about that asymmetric carbon are called anomers Cyclic form of glucose is a pyranose Cyclic form of fructose is a furanose Monosaccharides Exist in Cyclic, Anomeric Forms 7.2 What Is the Structure and Chemistry of Monsaccharides? Monosaccharides Can Be Converted to Several Derivative Forms • A variety of chemical and enzymatic reactions produce derivatives of the simple sugars • Some of the most common are: • Sugar acids • Sugar alcohols • Deoxy sugars • Sugar esters • Amino sugars • Acetals, ketals, and glycosides Monosaccharide Derivatives Reducing sugars are sugars with free anomeric carbons - they will reduce oxidizing agents, such as peroxide, ferricyanide and some metals (Cu2+ and Ag+). These redox reactions oxidize the sugar to a sugar acid. Glucose is a reducing sugar - so these reactions are the basis for diagnostic tests for blood sugar Monosaccharides Can Be Converted to Several Derivative Forms More Monosaccharide Derivatives • Sugar alcohols are formed by mild reduction of sugars • Deoxy sugars: constituents of DNA, etc. • Sugar esters: phosphate esters like ATP are important • Amino sugars contain an amino group in place of a hydroxyl group • Acetals, ketals and glycosides: basis for oligoand polysaccharides Sugar Alcohols Deoxy Sugars • Deoxy sugars are monosaccharides with one or more hydroxyl groups replaced by hydrogens • 2-Deoxy-D-ribose is a constituent of DNA • Rhamnose is a component of ouabain, a toxic “cardiac glycoside” • Fucose is a component of some cell walls Amino Sugars • Sugars with an amino group at C-2 are amino sugars. • They are found in many oligosaccharides and polysaccharides. Muramic acid Muramic acid is a component of the polysaccharides of cell membranes of higher organisms and also bacterial cell walls. Muramic acid is a glycosamine linked to a 3-carbon acid at C-3. (Murus is Latin for “wall”.) Sialic acids The N-acetyl and N-glycolyl derivatives of neuraminic acid are known as sialic acids. Acetals and Ketals Acetals and ketals can be formed from hemiacetals and hemiketals, respectively. Glycosides The pyranose and furanose forms of monosaccharides react with alcohols in dehydration synthesis reactions to form glycosides, with retention of the α- or βconfiguration at the C-1 carbon. The new bond formed is called a glycosidic bond. 7.3 What is the Structure and Chemistry of Oligosaccharides? • • • • Disaccharides are the simplest oligosaccharides Two monosaccharides linked by a glycosidic bond Each unit in an oligosaccharide is termed a residue Each of the structures in Figure 7.18 is a “mixed acetal”, with one hydroxyl provided intramolecularly and one hydroxyl from the other monosaccharide • Each of these (except for sucrose) possesses one free unsubstituted anomeric carbon, and is thus a reducing sugar • Sucrose is not a reducing sugar – it does not have a free anomeric carbon 7.3 What is the Structure and Chemistry of Oligosaccharides? 7.3 What is the Structure and Chemistry of Oligosaccharides? Know the important features • Be able to identify anomeric carbons and reducing and nonreducing ends • Note carefully the nomenclature of links. Be able to recognize alpha(1,4), beta(1,4) linkages, etc., in structures. 7.4 What is the Structure and Chemistry of Polysaccharides? • • • • • • Functions: storage, structure, recognition Nomenclature for polysaccharides is based on their composition and structure Homopolysaccharide – a polysaccharide that contains only one kind of monosaccharide Heteropolysaccharide – a polysaccharide made of several monosaccharides Starch and glycogen are storage molecules Chitin and cellulose are structural molecules Cell surface polysaccharides are recognition molecules Starch A plant storage polysaccharide • Two forms: amylose and amylopectin • Most starch is 10-30% amylose and 70-90% amylopectin • Branches in amylopectin every 12-30 residues • Amylose has alpha(1,4) links, one reducing end • The branches in amylopectin are α(1→6). Amylose and Amylopectin are energy storage molecules in plants The Structure of Amylose • Amylose is poorly soluble in water, but forms micellar suspensions • In these suspensions, amylose is helical • Iodine fits into the helices to produce a blue color Why Branching in Starch? Consider the phosphorylase reaction • Phosphorylase releases glucose-1-P products from the amylose or amylopectin chains • The more branches, the more sites for phosphorylase attack • Branches in amylopectin provide a mechanism for quickly releasing (or storing) glucose units for (or from) metabolism The Phosphorylase Reaction Releases Glucose Units for Metabolic Energy Glycogen The glucose storage device in animals • Glycogen constitutes up to 10% of liver mass and 1-2% of muscle mass • Glycogen is stored energy for the organism • Only difference from amylopectin: even more highly branched • Alpha(1,6) branches every 8-12 residues • Like amylopectin, glycogen gives a red-violet color with iodine Structural Polysaccharides • The composition of structural polysaccharides is similar to storage polysaccharides • But small structural differences greatly influence properties • Starch and glycogen linkages consist primarily of α(1→4) linkages. • Cellulose consists of β(1→4) linkages Cellulose Provides Physical Structure and Strength to Plants • • • • Cellulose is a structural polysaccharide It is the most abundant natural polymer in the world It is found in the cell walls of nearly all plants The wood and bark of trees are insoluble, highly organized structures formed from cellulose and lignin • Cotton is almost pure cellulose • Cotton acetates, made from the action of acetic anhydride on cellulose, are used in dresses, lingerie, and other clothing The Structural Difference Between Amylose and Cellulose (a) Amylose prefers a helical conformation (due to its bent α(1→4) linkages. (b) Cellulose, with β(1→4) linkages, can adopt a fully extended conformation. The Structure of Cellulose The structure of cellulose, showing the hydrogen bonds (blue) between the sheets, which strengthen the structure. Intrachain Hbonds in red; interchain Hbonds in green. Other Structural Polysaccharides • Chitin – found in the exoskeletons of crustaceans, insects and spiders, and cell walls of fungi • It is similar to cellulose, but C-2s are N-acetyl • Cellulose strands are parallel; chitins can be parallel or antiparallel Structures of cellulose, chitin and mannan Like cellulose, chitin and mannan form extended ribbons and pack together efficiently, taking advantage of multiple hydrogen bonds. Glycosaminoglycans – Linear Chains of Repeating Disaccharides Glycosaminoglycans are linear chains of repeating disaccharides in which one unit is an amino sugar and one or both is negatively charged. Functions of Glycosaminoglycans • Heparin, with a very high negative charge, is a natural anticoagulant. • Hyaluronates (consisting of up to 25,000 disaccharide units) are components of the vitreous humor of the eye and of synovial fluid, the lubricant fluid of the body’s joints • Chondroitins and keratan sulfate are found in tendons, cartilage, and other connective tissue • Dermatan sulfate is a component of the extracellular matrix of skin • Glycosaminoglycans are constituents of proteoglycans (discussed later in this chapter) Bacterial Cell Walls Composed of 1 or 2 bilayers and peptidoglycan shell • Gram-positive: One membrane phospholipid bilayer and thick peptidoglycan outer shell • Gram-negative: Two membrane phospholipid bilayers with thin peptidoglycan shell in between • Gram-positive: pentaglycine bridge connects tetrapeptides • Gram-negative: direct amide bond between tetrapeptides The Structure of Peptidoglycan The Structure of Gram-positive Peptidoglycan The Cell Wall of Gram-positive Bacteria The Cell Wall of Gram-negative Bacteria Animals Display a Variety of Cell Surface Polysaccharides • Animal cell surfaces contain an incredible diversity of glycoproteins and proteoglycans • These polysaccharide structures regulate cellcell recognition and interaction • The uniqueness of the "information" in these structures is determined by the enzymes that synthesize these polysaccharides 7.5 What Are Glycoproteins, and How Do They Function in Cells? • May be N-linked or O-linked • N-linked saccharides are attached via the amide nitrogens of asparagine residues • O-linked saccharides are attached to hydroxyl groups of serine, threonine or hydroxylysine The Structure of O-Linked Saccharides O-linked Saccharides of Glycoproteins The O-linked saccharides often adopt extended conformations to lift the functional domains of these proteins above the membrane surface. The Structure of N-Linked Saccharides Figure 7.32 The carbohydrate moieties of glycoproteins may be linked to the protein via asparagine residues (in N-linked saccharides). The Structure of N-Linked Saccharides Figure 7.32 N-linked glycoproteins are of three types: high mannose, complex, and hybrid, the latter of which combines structures found in the high mannose and complex saccharides. Functions of N-linked Oligosaccharides Many functions known or suspected • Oligosaccharides can alter the chemical and physical properties of proteins • Oligosaccharides can stabilize protein conformations and/or protect against proteolysis • Cleavage of monosaccharide units from Nlinked glycoproteins in blood targets them for degradation in the liver Structures of Proteoglycans Structure of rat cartilage matrix proteoglycan. This molecule is highly hydrated. Compression of cartilage (as in walking) squeezes water out of the cartilage tissue to cushion the joint. Water is reabsorbed when the stress and compression diminishes. Lectins are proteins that bind carbohydrates with high specificity and high affinity Sugars are the “letters” of the sugar code. Lectins are the translators of the sugar code. Many processes, such as cell migration, cell-cell interactions, immune responses, and blood clotting, depend on information transfer using this code.