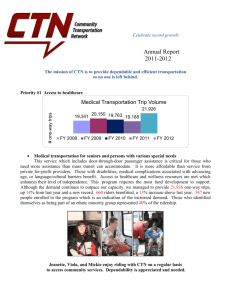
BMT CTN: Numbers of Protocols Opened
advertisement

Blood and Marrow Transplant Clinical Trials Network (BMT CTN): Past, Present and Future Hillard M. Lazarus, MD The George & Edith Richman Professor and Distinguished Scientist in Cancer Research Director of Novel Cell Therapy University Hospitals Case Medical Center Case Western Reserve University E Donnall Thomas, MD 1920-2012 Nobel Prize in Physiology or Medicine, 1990 BONE MARROW TRANSPLANTATION Initial Report: Mary Imogene Bassett Hospital ED Thomas, et al. N Engl J Med 257: 491-496, 1957 • N=6 pts: variety of diseases, malignant & non-malignant • differing marrow products infused • demonstrated safety (no marrow emboli) • demonstrated some donor engraftment F Appelbaum. N Engl J Med 357: 1472-1475, 2007 Genesis I can’t cover everything BLOOD AND MARROW TRANSPLANT CLINICAL TRIALS NETWORK Established November 2001 Mission: Conduct scientifically meritorious multicenter trials in an efficient manner to improve transplant outcomes Provide infrastructure to allow promising therapies to be developed/evaluated in high quality, multicenter studies that give definitive answers as rapidly as possible BMT CTN Organizational Structure NHLBI & NCI Data and Safety Monitoring Board Protocol Review Committee NCI Coop Group Chairs (ex officio) STEERING COMMITTEE Administrative Committees Technical Committees Data and Coordinating Center 20 Clinical Cores; High-performing Affiliate Centers Protocol Teams Affiliate Clinical Centers BMT CTN Data and Coordinating Center CIBMTR Electronic Communications Statistical Overall Design/ Coordination Data Analysis Management Scientific Protocol Leadership Trial Oversight/ Development/ Monitoring Implementation Lab/ Medical Repository Monitoring Management Patient Advocacy * EMMES NMDP Contracting * professional partner to clinicians, scientists, program leaders BLOOD & MARROW TRANSPLANTATION 1st State of Science Symposium: 4/01/2000 • Stem cell source & donor selection – Horowitz, Champlin, Anasetti, Hansen, Wagner, Confer • Regimen-related toxicity – Armitage, Blume, McDonald, Jones • Graft-versus-host disease – Blazar, Martin, Guinan, Parkman, Storb, Ferrara • Recurrence after autograft – Nadler, Vose, Press, Gribben, Antman BLOOD & MARROW TRANSPLANTATION 1st State of Science Symposium: 4/01/2000 • Recurrence after allograft – O’Reilly, Scheinberg, Barrett, Levitsky, Riddell • Infectious complications – Wingard, Forman, Zaia, Heslop • Late complications & immune recovery – Sullivan, Weinberg, Gress, Vogelsang BLOOD & MARROW TRANSPLANTATION 1st State of Science Symposium Conclusions: 1. Necessity of multi-institutional studies 2. Studies can be adapted to multi-institutional setting 3. Studies could be completed in a responsible time BLOOD & MARROW TRANSPLANTATION 1st State of Science Symposium Conclusions for studies needed: 1. Blood vs marrow in matched sibling donors – (NA) 2. Blood vs marrow in matched unrelated donors – (0201) 3. Techniques to improve cord blood engraftment – (0501) 4. T-cell depletion studies – (0303) 5. Methods to improve autologous cell collection – (NA) 6. Comparisons of related and unrelated HCT vs standard chemotherapy for high risk patients – (S1203) BMT CTN FOUNDATION Creation and Organization/Administration RFA from NHLBI in 2001 Competition for Data Coordinating Center (DCC) Emmes Corp, NMDP and CIBMTR awarded Competition for Centers Established 16 Core Centers Case Consortium original Core Center ( Re-competition: expanded to 20 in July 2011 ) BMT CTN Case Consortium (Original & Current) Case Western Reserve University (CWRU); Oregon Health Sciences University (OHSU); University of Illinois Chicago Transition to add Washington University (St. Louis) and Ohio State University (through 2011) Present configuration CWRU, OHSU Cleveland Clinic, West Virginia University BMT CTN FOUNDATION Creation and Organization/Administration Formation of committees and teams: Manual of Policies/Procedures (MOP) Disease-specific teams Protocol-specific teams Liaison relation with cooperative oncology groups Electronic data capture system Per patient reimbursement model Websites for members & public Metrics for center performance: “Report Card” Protocol Development & Prioritization Clinical Population Scientific Rationale Feasibility Issues Logistical/ budgetary Constraints Collaboration with cooperative groups to avoid duplication C No. pts 440 1,058 2001 2002 2003 2004 2005 2006 1,615 2,133 2007 2008 2625 3048 4,200 2009 2010 2011 5,200 2012 2013 1101 Haplo vs. 2 UCB 0903 Allo Tx for HIV+ 0901 Full vs RIC - MDS/AML 0804 High Risk CLL 0902 Post Tx Stress Mgmt 0803 HIV+ Lymphoma 0702 PIII Myeloma Follow-on 0801 P II/III CGVHD Treatment 0802 PIII AGVHD Treatment 0701 PII NST for NHL 0604 PII DCB in Adult 0603 PII Haplo in Adult 0601 PII Sickle Cell NST 0703 PII HD 0403 PIII Etanercept for IPS 0704 PIII MM maintenance 0502 PII NST for AML >60y 0402 PIII GVHD prophylaxis = Enrollment complete 0501 III Single vs Double CBT = Enrollment on-going = Cumulative actual [projected] accrual 0301 PII Unrelated Tx for aplastic anemia 0401 PIII BEAM vs BEAM-Bexxar for Lymphoma 0302 PII AGVHD therapy 0303 PII T-depleted HCT for AML = Coop group collaboration 0202 PIII follicular NHL (closed early) (see color key above) 0201 PIII Unrelated PBSC vs. Marrow 0101 PIII Vori vs. Fluconazole 0102 PIII Myeloma Tandem HCT (closed early) BMT CTN Centers, 2013 >115 centers enrolled >5000 pts since 2003 = Core Centers = PBMTC Centers = Affiliate Centers Mmh11_9.ppt 8 BMT CTN: Numbers of Protocols Opened 0702 0803 0801 0804* 0802 0805* 7 6 5 0302 0303 0401 4 3 2 1 0301 0403 0402 0502* 0501 0704* 0601 0603 0604 0703* 0101 0201 0102 0202 1102 1202 1203 1204 1205 0901 0903 0902 1101 0701 0 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 (proj) BMT CTN Yearly & Cumulative Accrual All Protocols, 2004-2012 1200 6000 1000 5000 800 4000 600 3000 400 2000 200 1000 0 0 2004 2005 2006 2007 2008 2009 2010 2011 2012 BMT CTN TRIALS Protocol Categories All Trials Phase II Donor/Graft Source Phase III 12 6 6 GVHD 5 3 2 Infection 3 2 1 Disease Control 12 6 6 Regimen Toxicity 4 2 2 QOL 7 2 5 28 14 14 TOTAL BMT CTN Publications Summary 2012: 7 peer-reviewed papers (+1 in press) 28 total: 10 primary results papers: 0101, 0102, 0202, 0301, 0302, 0303, 0401, 0601, 0603/0604, 0704 (100104) 8 other protocol-related papers 3 methodology papers 7 other Network publications BLOOD & MARROW TRANSPLANTATION 2nd State of Science Symposium - BMT CTN organized and led June 7-8, 2007 @ Ann Arbor, MI Goal: identify key transplant-related issues Propose critical trials to address these issues - may be sequential phase II III - may require cooperative oncology group or other participants - trials should be ready to start quickly BLOOD & MARROW TRANSPLANTATION 2nd State of Science Symposium 1. Optimal donor and graft source – C. Anasetti 2. Regimen-related toxicity – E. Stadtmauer 3. Graft-versus-host disease – J. Antin 4. Infection & immune reconstitution – J. Wingard 5. Late effects/quality of life – S. Lee 6. Pediatrics – K. Schultze, J. Levine BLOOD & MARROW TRANSPLANTATION 2nd State of Science Symposium (con’t) 7. Leukemia – F. Appelbaum 8. Lymphoma – R. Negrin 9. Plasma cell myeloma – S. Giralt 10. Non-malignant disorders – C. Bredson 11. Gene and cell therapy – H. Heslop, D. Kohn 12. Cinical trial design – M. Horowitz BLOOD & MARROW TRANSPLANTATION Timeline: 2nd State of Science Symposium April 2006 June-Dec 2006 Dec 2006 Feb 2007 May 2007 June 2007 Committees named and charged Committee conference calls Committee in-person mtgs @ ASH Committee in-person mtg @ Tandem Document due SOSS JL Ferrara, BMT CTN. Biol Blood Marrow Transplant 13: 1268-1285, 2007 BLOOD & MARROW TRANSPLANTATION 2nd State of Science Symposium: Conclusions 1. GVHD: Phase II trial calcineurin-free regimen – 0402 2. QOL: Phase III study stress management – 0902 3. Myeloma: Phase III comparison tandem HCT vs. consolidation and maintenance – 0702 4. AML: Phase III chemotherapy vs. URD HCT – SWOG 1203 5. AML: Phase III full intensity vs. RIC HCT - 0901 6. Ph+ ALL: Phase III chemotherapy vs. Allo HCT – S0805 BLOOD & MARROW TRANSPLANTATION 2nd State of Science Symposium: Conclusions 7. CLL: Phase II RIC Allo HCT for high risk CLL – 0804 8. Lymphoma: Phase II RIC Allo HCT in T cell lymphoma – NA 9. HLH: Phase II RIC for children with HLH – planning 10. Non-malignant: Phase II auto HCT in Crohn disease – NA 11. Cell Therapy: Phase II viral-specific CTL adenovirus - NA MAJOR SCIENTIFIC PUBLICATIONS Potentially Practice-Changing BMT CTN Donor Graft Source Questions Blood versus Marrow Extremely complex undertaking; Dual consent: donor and recipient BLOOD vs MARROW GRAFT SOURCE Matched Unrelated Donors (MUD): Engraftment C Anasetti, BMT CTN. N Engl J Med 367: 1487-96, 2012. BLOOD vs MARROW GRAFT SOURCE Matched Unrelated Donors (MUD): GVHD C Anasetti, BMT CTN. N Engl J Med 367: 1487-96, 2012. BLOOD vs MARROW GRAFT SOURCE Matched Unrelated Donors (MUD): Relapse & TRM C Anasetti, BMT CTN. N Engl J Med 367: 1487-96, 2012. BLOOD vs MARROW GRAFT SOURCE Matched Unrelated Donors (MUD): Survival C Anasetti, BMT CTN. N Engl J Med 367: 1487-96, 2012. BMT CTN 0401 Reduce Relapse After Autograft: NHL DLBCL: BEXXAR-BEAM vs Rituximab-BEAM No differences in patient outcome except increased mucositis Randomized to Bexxar/BEAM or Rituxan/BEAM Within 3 mo of mobilization Day -19 Bexxar 5 mCi dosimetry Rituxan 375 mg/m2 Day -12 Bexxar 75 cGy TBD Rituxan 375 mg/m2 Day -6 Days -5 to -2 Days -5 to -2 Day -1 Day 0 Day +5 BCNU 300 mg/m2 Etoposide [VP-16] 100 mg/m2 BID (8 doses) Ara-C [Cytarabine] 100 mg/m2 BID (8 doses) Melphalan 140 mg/m2 Infusion of mobilized hematopoietic cells G-CSF 5 µg/kg daily until ANC >500/mm3 x 3 days BMT CTN 0401 Autograft BEXXAR-BEAM vs Rituximab-BEAM Probability Progression-free survival (PFS) Probability, % 100 100 90 90 80 80 70 70 Rituxan/BEAM (N=113) 60 60 50 50 Bexxar/BEAM (N=111) 40 40 30 30 20 Bexxar/BEAM @ 2 yr: 48.6% Rituxan/BEAM @ 2 yr: 49.0% 10 20 p=0.65 10 0 0 0 6 12 18 24 30 36 42 Months JM Vose, BMT CTN. J Clin Oncol 31: 1662-1668, 2013. 48 BMT CTN 0401 Autograft BEXXAR-BEAM vs Rituximab-BEAM Probability Survival Probability, % 100 100 90 90 80 80 70 70 Rituxan/BEAM (N=113) 60 60 50 50 Bexxar/BEAM (N=111) 40 40 30 30 20 Bexxar/BEAM @ 2 yrs: 60.1% Rituxan/BEAM @ 2 yrs: 66.3% 10 20 p=0.29 10 0 0 0 6 12 18 24 30 36 42 Months JM Vose, BMT CTN. J Clin Oncol 31: 1662-1668, 2013. 48 BMT CTN Relapse Prevention Questions: Myeloma Tandem Autograft vs Autograft-Allograft TRANSPLANTATION IN MYELOMA Tandem Autograft vs Autograft-RIC Allograft Autografts: Melphalan 200 mg/m2 Allograft: TBI 200 cGy A Krishnan, BMT CTN. Lancet Oncol 12: 1195-203, 2011. TRANSPLANTATION IN MYELOMA Tandem Autograft vs Autograft-RIC Allograft Standard-risk A Krishnan, BMT CTN. Lancet Oncol 12: 1195-203, 2011. TRANSPLANTATION IN MYELOMA Tandem Autograft vs Autograft-RIC Allograft High-risk A Krishnan, BMT CTN. Lancet Oncol 12: 1195-203, 2011. BMT CTN Relapse Prevention Questions: Myeloma Post-Autograft Maintenance Therapy AUTOGRAFT IN MYELOMA Post-Transplant Lenalidomide vs Placebo Joint BMT CTN and CALGB study 88% @ 3 yr Median TTP 46 mo 80% @ 3 yr Median TTP 27 mo PL McCarthy, BMT CTN. N Engl J Med 366: 1770-81, 2012. AUTOGRAFT IN MYELOMA Post-Transplant Lenalidomide vs Placebo Risk 2nd maligancy PL McCarthy, BMT CTN. N Engl J Med 366: 1770-81, 2012. BMT CTN Novel GVHD Prevention Strategies T Cell Depletion and Other Strategies HEMATOPOIETIC CELL TRANSPLANT Allograft & High-dose Rituximab in FCC NHL Survival Graft-vs-Host Disease IF Khouri, MD Anderson. Blood 111: 5530-5536, 2008 BMT CTN 0701 Allograft & High-dose Rituximab in FCC NHL Rituximab 375 mg/m2 Rituximab 1,000 mg/m2 (day –13) (day – 6) Fludarabine + Cyclophosphamide conditioning Tacrolimus+ Methotrexate (GVHD prophylaxis) Rituximab 1,000 mg/m2 day +1 and +8 Blood allograft infusion (matched-related or MUD) PK studies for rituximab blood concentration Accrual completed: awaiting DSMB recommendations GRAFT-VS-HOST DISEASE Prophylaxis: T Cell Depletion • Decades of failure • Engraftment failure • Prolonged immune incompetence • viral, opportunistic infections • High relapse rates VT Ho, RJ Soiffer. Blood 98: 3192-204, 2001. GRAFT-VS-HOST DISEASE Prophylaxis: AML CR1 & T Cell Depletion • Multi-center BMT CTN trial: CD34 selection, Miltenyi device • Few AML CR2; early follow-up Grade 3-4 Acute GVHD Chronic GVHD S Devine, BMT CTN. Biol Blood Marrow Transplant 17: 1343-51, 2010 GRAFT-VS-HOST DISEASE Prophylaxis: AML CR1 & T Cell Depletion Relapse Survival No engraftment failures S Devine, BMT CTN. Biol Blood Marrow Transplant 17: 1343-51, 2010 AML CR1 ALLOGRAFTS: BMT CTN T Cell Depletion vs Immune Suppression N=44 T Cell Depletion; N=88 Immune Suppression Relapse Survival GVHD-Free Survival MC Pasquini, BMT CTN. J Clin Oncol 30: 3194-3201, 2012 GRAFT-VS-HOST DISEASE Prophylaxis: Combination Myeloablative conditioning: Cy or VP-16 plus TBI > 1200 cGy Mobilized blood graft Median (range) age 44 (13-59) yr C Cutler, BMT CTN. Blood 120: 2012 (abstract #739). BMT CTN GVHD PROPHYLAXIS Sirolimus/Tacrolimus vs Tacrolimus/Methotrexate Sirolimus/tacrolimus: No advantage in 114-day acute GVHD-free survival • 2 and 3 days faster neutrophil and platelet engraftment • Reduction in acute GVHD 8% absolute II-IV, p = 0.17 7% absolute III-IV, p = 0.05 • More chronic GVHD 9% absolute , p = 0.05 • Less mucositis but more endothelial injury (all p 0.05) Acceptable alternative to tacrolimus/methotrexate BMT CTN Regimen-Intensity Questions Acute Leukemia and MDS ACUTE MYELOID LEUKEMIA CR1 Reduced-Intensity Conditioning in Elderly ACUTE MYELOID LEUKEMIA CR1 Reduced-Intensity Conditioning in Elderly • • • • • CALGB & BMT CTN: N=123 @ 21 centers Median age 65 (60-74) yr N= 58 matched-related donor; N=65 MUD 82 intermediate cytogenetics; 25 adverse cytogenetics Fludarabine + busulfan ± ATG Event Treatment-related mortality Acute GVHD gr 3-4 Chronic GVHD Relapse Overall survival Incidence @ 2 Yr 14% 3.4% @ 100 days 26% 47% 46% SM Devine, CALGB, BMT CTN. Blood 120: 2012 (abstract #230). ACUTE MYELOID LEUKEMIA CR1 Prospective Randomized: RIC vs Myeloablative German AML Study Group: small series M Bornhäuser, German AML. Lancet Oncol 13: 1035-1044, 2012. ACUTE MYELOID LEUKEMIA & ALLOGRAFT Myeloablative vs Reduced-Intensity Conditioning BMT CTN 0901 BMT CTN Alternative Donor Graft Source Questions No Matched-Related or MUD Available GRAFT-VS-HOST DISEASE Post-Transplant Cyclophosphamide Acute GVHD Chronic GVHD N=117; Bu/CY T-replete marrow CY 50 mg/kg/d T+3, T+4 L Luznik, Hopkins. Blood 115: 3224-30, 2010. BMT CTN Protocol 1101 Multi-center, Phase III, Randomized Trial of Reduced Intensity Conditioning and Transplantation Of Double Unrelated Umbilical Cord Blood versus HLA-Haploidentical Related Bone Marrow for Patients with Hematologic Malignancies Followup to 2 independent BMT CTN phase II studies BMT CTN 0603 and 0604 31% 54% CG Brunstein, BMT CTN. Blood 118:282-288, 2011 45% BMT CTN Study Design Protocol 1101 Patient ≥ 18 and ≤70 yr Acute leukemia or lymphoma Adequate organ function Performance score ≥70 No sibling or matched unrelated donor available, BUT: • Double umbilical cord blood (UCB) graft • Haploidentical related donor marrow (Haplo-BM) • No donor specific anti-HLA-Ab Randomization Stratified by Transplant Center Double UCB Haplo-BM BMT CTN Protocol 1101 Minnesota Protocol (0604) Haploidentical Hopkins Protocol (0603) Eliminate alloreactive T cells BMT CTN Manuscripts in Preparation 0402 – Sirolimus vs methotrexate (in combo with tacrolimus) to prevent acute GVHD : NO BENEFIT 0403 – Etanercept for Idiopathic Pneumonia Syndrome: NO BENEFIT 0501 – Single vs double UCB transplant in children: MORE GVHD with double; NO ADVANTAGE engraftment or survival 0502 – RIC HCT for older AML adults: GOOD RESULTS 0802 – MMF as initial therapy for AGVHD: NO BENEFIT BMT CTN Future • Continued accrual enhancement • Continued publications in high-impact journals • Repository trials: GVHD blood biomarkers BMT CTN Protocol 1202 Prospective Multi-Center Cohort for the Evaluation of Biomarkers Predicting Risk of Complications and Mortality Following Allogeneic HCT 1,500 allogeneic patients over 4 yr: HCT only @ US centers Samples collected for DNA, RNA, and proteins: R24 Building upon University of Michigan data and other sources Data collection for post-transplant complications BMT CTN Protocol 1202 Recipient Samples Biomarker Approach Genetic Proteomic Gene Expression Sample DNA 17mL blood Serum (10 mL blood) PAXgene Lysates(20 mL blood) No. Pts 1500 1500 240 Days post-HCT Pre-HCT PreDay -1 or 7 14 ± 21 28 42 Conditioning 0 ±2 2 ±2 ±2 ±3 56 90 ± 3 ±10 X X X X X X X X X X X BMT CTN Future (con’t) • Partnering with other groups: • IFM • Canadians • Germans • Increased companion translational trials: obesity • 3rd State of the Science Symposium IFM/DFCI 2009 Phase III: Untreated Myeloma • Symptomatic myeloma with measurable disease <65 yrs and transplant-eligible; ECOG <2 (KPS ≥60%) Initial Therapy RVD Cycle 1 R A N D O M I Z E Arm A RVD Cycles 2-3 HD Cytoxan; collect cells RVD Cycles 4-8 Maintenance lenalidomide (Melphalan + HCT @ relapse) Arm B RVD Cycles 2-3 HD Cytoxan; collect cells HD Melphalan + HCT RVD=lenalidomide; RVD for 2 more cycles bortezomib; Maintenance Lenalidomide dexamethasone 1° Endpoint: PFS 2° Endpoints: relapse, TTP, survival, QOL, economics, genetic prognostic BMT CTN Future (con’t) • Partnering with other groups: • IFM • Canadians • Germans • Increased companion translational trials: obesity • 3rd State of the Science Symposium COOPERATIVE ONCOLOGY GROUPS Obesity and Myeloma: BMT CTN 0702 Myeloma patients within 9 months of diagnosis Single autograft +/- consolidation versus tandem autograft and maintenance N=750 patients (250 each arm) Uniformity of treatment: Melphalan 200 mg/m2 IV plus autograft Accrual nearly reached COOPERATIVE ONCOLOGY GROUPS Companion Investigation: Example Myeloma is an obesity-driven disease Critical questions - What is impact of obesity on treatment and disease? - If obesity has detrimental effects, what are the mechanisms & how can these be addressed & improved? - If obesity has beneficial effects, how can these be identified and used to enhance therapeutic outcomes? OBESITY AND MYELOMA Limitations of BMI (body mass index) Anthropomorphic measures of abdominal adiposity correlate with cardiovascular and cancer mortality: independent of BMI Which anthropomorphic measurements are better? Waist:Hip measure better indicator of visceral fat better correlation with incidence colon & ovarian cancer C Zhang, et al. Circulation 117: 1658-1667, 2008 YC Wang, et al. Obesity 15: 2855-2865, 2007 OBESITY AND MYELOMA Opportunity to study prospectively other biologic measurements Identify mechanisms by which obesity impacts therapy Identify mechanisms by which obesity affects disease progression Identification of potential markers and mediators to impact disease progression COOPERATIVE ONCOLOGY GROUPS Clinical Investigation: Example Companion translational obesity study to transplant trial Investigators: HM Lazarus E Campagnaro NA Berger Anthropomorphic measures at frequent intervals Analysis of prospectively collected/archived blood samples Measure Hip & Waist Circumference OBESITY AND TRANSPLANT Myeloma: BMT CTN 0702 Waist:Hip measurement Plasma biomarkers Adipokines/cytokines: • adiponectin, leptin, IL-6, TNF-α Hormones: • insulin, C-peptide, pancreatic peptide (PP), peptide YY (PYY) Growth factors: • IGF-1, IGFBP-3 BMT CTN Future (con’t) • 3rd State of the Science Symposium • February 24-25, 2014 @ Grapevine, TX