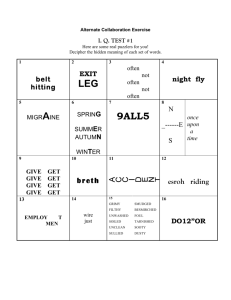
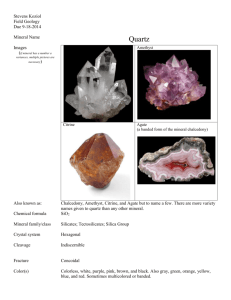
Introduction to Environmental Geochemistry - FAU
advertisement

Color in Minerals GLY 4200 Fall, 2012 1 Color Sources • Minerals may be naturally colored for a variety of reasons - among these are: Selective absorption Crystal Field Transitions Charge Transfer (Molecular Orbital) Transitions Color Center Transitions Dispersion 2 Characteristic Color • Color is characteristic for some minerals, in which case it is idiochromatic and thus may serve as an aid to identification • Color is often quite variable, which is called allochromatic, and thus may contribute to misidentification 3 Visible Light • Visible light, as perceived by the human eye, lies between approximately 400 to 700 nanometers 4 Interaction of Light with a Surface • Light striking the surface of a mineral may be: Transmitted Refracted Absorbed Reflected Scattered 5 Absorption • Color results from the absorption of some wavelengths of light, with the remainder being transmitted • Our eye blends the transmitted colors into a single “color” 6 Mineral Spectrum • Spectrum of elbaite, a tourmaline group mineral • Note that absorbance is different in different directions 7 • What color is this mineral? Crystal Field Splitting • Partially filled 3d (or, much less common, 4d or 5d) allow transitions between the split d orbitals found in crystals • The electronic configuration for the 3d orbitals is: 1s2 2s2 2p6 3s2 3p6 3d10-n 4s1-2, where n=1-9 8 Octahedral Splitting • Splitting of the five d orbitals in an octahedral environment • Three orbitals are lowered in energy, two are raised • Note that the “center position” of the orbitals is unchanged 9 Tetrahedral Splitting • Tetrahedral splitting has two orbitals lowered in energy, while three are raised 10 Square Planar Splitting • a) octahedral splitting • b) tetragonal elongation splits the degenerate orbitals • c) total removal of ions along z axis produces a square planar environment 11 Factors Influencing Crystal Field Splitting • Crystal Field Splitting (Δ) is influenced by: Oxidation state of metal cation – Δ increases about 50% when oxidation state increases one unit Nature of the metal ion – Δ3d < Δ4d < Δ5d About 50% from Co to Rh, and 25% from Rh to Ir Number and geometry of ligands Δo is about 50% larger than Δt 12 Absorption Spectra of Fe Minerals 13 Emerald and Ruby Spectra • The field around Cr3+ in ruby is stronger than in beryl • Peaks in emerald are at lower energy 14 Emerald and Ruby Photos 15 Charge Transfer • Delocalized electrons hop between adjacent cations • Transition shown produces blue color in minerals such as kyanite, glaucophane, crocidolite, and sapphire 16 Sapphire Charge Transfer • Sapphire is Al2O3, but often contains iron and titanium impurities • The transition shown produces the deep blue color of gem sapphire 17 Sapphire 18 Sapphire Spectrum • Sapphires transmit in the blue part of the spectrum 19 Fluorite Color Center • An electron replaces an Fion 20 Fluorite • Grape purple fluorite, Queen Ann Claim, Bingham, NM. 21 Smoky Quartz • Replacement of Si4+ with Al3+ and H+ produces a smoky color 22 Smoky Quartz and Amythyst 23 Quartz, variety Chrysoprase • Green color usually due to chlorite impurities, sometimes to admixture of nickel minerals 24 Milky Quartz • Milky quartz has inclusions of small amounts of water 25 Rose Quartz • Color often due to microscopic rutile needles 26 Blue Quartz 27 Rutilated Quartz 28 Quartz, variety Jasper • Color due to admixture of hematite in quartz 29