ppt
advertisement
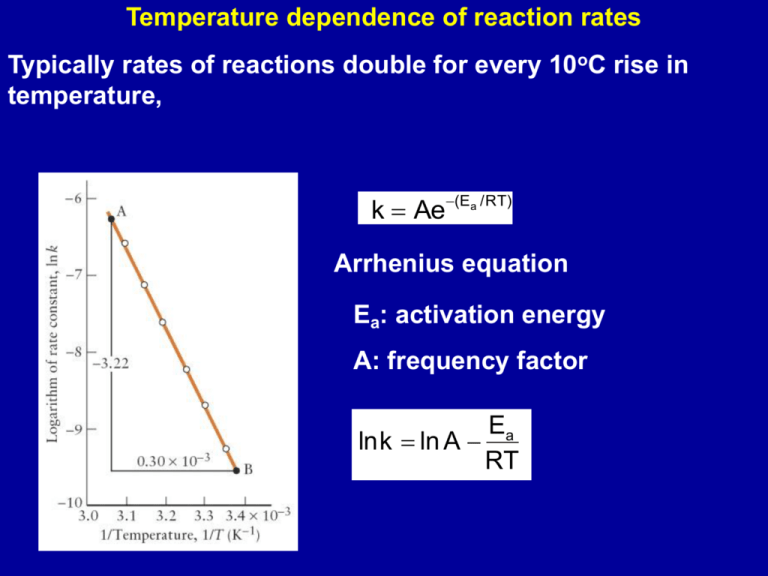
Temperature dependence of reaction rates Typically rates of reactions double for every 10oC rise in temperature, k Ae(Ea /RT) Arrhenius equation Ea: activation energy A: frequency factor Ea lnk ln A RT An Arrhenius plot of ln k against 1/T is used to determine Ea and A The higher the Ea the stronger the temperature dependence of the rate constant Collision Theory Collisions between two (or more) atoms/molecules required for a reaction. However, every time two reactants collide they may not react As temperature increases: atoms/molecules collide more frequently kinetic energy of atoms/molecules increases Collision theory: reaction occurs only if the reactants collide with a kinetic energy of at least the activation energy, and they do so in the correct orientation. Kinetic energy is important Orientation is important Cl N O 2 AB -> A2 + B2 2 NOCl 2 NO + Cl2 Animation 1 Animation 2 Animation 3 k Ae(Ea /RT) Ea lnk ln A RT The factor e-Ea/RT: fraction of molecules that have at least the minimum energy required for reaction. For an Ea = 40 kJ/mol Temperature (K) e-Ea/RT 298 9.7 x 10-8 400 5.9 x 10-6 600 3.3 x 10-4 A: reflects orientation effect or steric effect Measuring k as a function of T Ea to be determined k2 Ea 1 1 ln ( ) k1 R T2 T1 Reaction coordinate diagram Activated complex or transition state - highest energy along reaction coordinate Reactants must collide with sufficient energy to reach this point and collide in a preferred orientation to form the activated complex DE = (Ea)forward - (Ea)reverse Higher temperatures favor products for an endothermic reaction and reactants for an exothermic reaction Endothermic reaction: Ea(forward) > Ea(reverse) Exothermic reaction: Ea(forward) < Ea(reverse) CH3OH(aq) + H+(aq) CH3OH2+(aq) CH3OH2+(aq) + Br- (aq) CH3Br + H2O(aq) Catalysis Catalyst: a compound which speeds up the rate of a reaction, but does not itself undergo a chemical change. Simple mechanism A + catalyst intermediates intermediates B + catalyst Overall: AB Concentration of catalyst is included in k; hence k varies with concentration of catalyst Presence of a catalyst provides an alternate path with a lower Ea 2H2O2(aq) 2H2O(aq) + O2(g) In the absence of a catalyst, Ea = 76 kJ/mol In the presence of a catalyst (I-); Ea = 57 kJ/mol; rate constant increases by a factor of 2000 H 3C CH3 C H H (g) C H cis-2-butene CH3 C H 3C C (g) H trans-2-butene Catalyzed by I2 Pt C2H4(g) + H2(g) C2H6 (g) Example of heterogenous catalysis A catalyst does not effect the thermodynamics of the reaction DG is not affected by catalyst; neither is K Equilibrium concentrations are the same with and without catalyst; just the rate at which equilibrium is reached increases in the presence of a catalyst K = k1/k-1; catalyst speeds up both the forward and reverse reaction Enzymes Practically all living reactions are catalyzed by enzymes; each enzyme specific for a reaction. Enzymes typically speed up rates by 107 - 1014 times rate of uncatalyzed reactions Ea for acid hydrolysis of sucrose: 107 kJ/mol Ea for catalyzed acid hydrolysis of sucrose: 36 kJ/mol Rate increase of 1012 at body temperature E + S ES ES P + E “Poisoning” a catalyst Arsenic poisoning: Ingestion of As(V) as AsO43- results in reduction to As(III) which binds to enzymes, inhibiting their action Nerve gases - block enzyme-controlled reactions that allow nerve impulses to travel through the nerves. Catalytic Converters Incomplete combustion of gasoline produces CO, hydrocarbon fragments (CmHn) High temperature in the engine causes oxidation of N2 to NO and NO2 Conversion of these pollutants to less harmful compounds is speeded up in the presence of catalysts. 2 NO(g) catalyst CO, CmHn, O2 N2(g) + O2(g) catalyst CO2, H2O Catalyst: pellets of Pt, Pd, Rh animation






