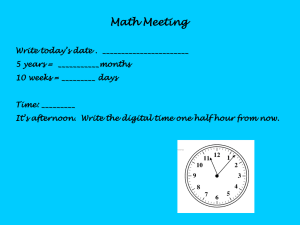
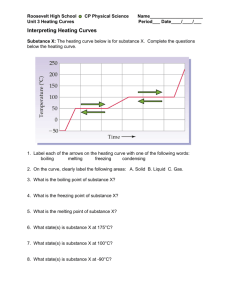
How Do Heating Curves Illustrate Phases of Matter? Here is a graph
advertisement

How Do Heating Curves Illustrate Phases of Matter? Here is a graph with an odd step-like shape: We are going to learn why it has that shape and what occurs during the different steps on the graph. 1.) Based on its shape, do you think the graph illustrates heating something up or cooling something off? ________________________________ 2.) Go to this web-site: http://www.kentchemistry.com/links/Matter/HeatingCurve.htm. Watch the short video embedded in the page and then answer the following questions about the step-like graph. a. What is happening during segment AB? __________________________________________________ b. What is happening during segment BC? __________________________________________________ c. What is happening during segment CD? _________________________________________________ d. What is happening during segment DE? _________________________________________________ e. What is happening during segment EF? _________________________________________________ f. During which segment(s) of the graph is only ONE PHASE present? _________________________ g. During which segment(s) of the graph are TWO PHASES present? __________________________ h. Why do you think the graph levels out (plateaus) during a phase change? What is the heat used for if not to make the temperature rise??? _____________________________________________________ If you need a hint, you may also look at the graph that resembles yours below the video. Now go to this web-site: http://www.kentchemistry.com/links/Matter/PhaseChangesA.htm . Click on each of the substances to melt them and fill in the chart below. Substance Greyish solid Melting Point (˚C) Boiling Point (˚C) Purple solid Green solid OVER How Do Heating Curves Illustrate Phases of Matter? Now we will make our own heating curves for 2 different substances and label all the steps. Go to this web-site: http://nces.ed.gov/nceskids/createagraph/default.aspx. 1. Select “XY graph” , then select the “Data” tab on the side. 2. In the “Data Set” box, select 16 data points, and 2 groups of data. Create one graph with 2 lines on it using the following data. Pls put time on the x-axis, and temp on the y-axis. 3. Save the graph as a JPG file. You will have to label some things on your graph. To do so, you can either print it & write on it, or paste it into a word document, type on it and then print it. Heating of Water Heating of Ethanol Time (min) Time (min) Temp (˚C) Temp (˚C) 0 -110 0 -227 1 -100 1 -217 2 -75 2 -192 3 -50 3 -167 4 -25 4 -142 5 0 5 -117 6 0 6 -117 7 0 7 -117 8 25 8 -52 9 50 9 13 10 75 10 65 11 100 11 78 12 100 12 78 13 100 13 78 14 125 14 103 15 150 15 128 4. Label each step on your graph. You should use the following words (some used more than once): solid, liquid, gas, melting, boiling. 5. Fill in the blanks in the chart below using your curves for water and ethanol. Substance Melting Point (˚C) Boiling Point (˚C) Water Ethanol 6. Below draw a cooling curve for water. Label each step using the following words (some used more than once): solid, liquid, gas, condensing, freezing. OVER