Heat Exchange During Physical Changes - Parkway C-2
advertisement

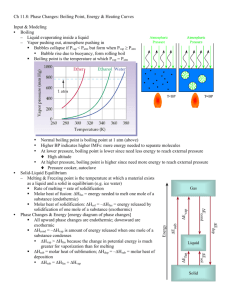
Heat Exchange During Physical Changes Heating Curve: graph of temperature vs. time (or heat added) As a substance is heated, (imagine a beaker of water in this case) its temperature only rises when no phase change is occurring. Why is this? Answer: the phase change requires (or releases) energy. Example: boiling water is endothermic, so any heat added during the boiling process is used for boiling, not raising the temperature. The same can be said for melting. The opposite can be said for condensing and freezing, because they are exothermic. Therefore, we can say that every sample of water that contains at least a little liquid should be between ____ and ______oC. Question: Look at the parts of the curve in which the temperature is changing (3 separate places). What causes the differences in the slopes? Calculating heat exchanged during a phase change: Heat of fusion (Hfus.): Amount of heat released as liquid freezes to solid or heat absorbed as solid melts to liquid q = m x Hfus. try: How much heat is absorbed by 25.6 g of ice as it melts? q = m x Hfus. = 25.6 g x 334 J/g = 8550 J Heat of vaporization (Hvap.) heat released as gas condenses to liquid or heat absorbed as liquid vaporizes to gas q = m Hvap. Try these problems: How much heat is released as 15.6 g of ethyl alcohol (ethanol) condenses? 13700 J The cooling tower in a nuclear power plant utilizes the process of water vaporization to cool hot pipes from inside the plant. If the heat that must be released is 4.56 megajoules per hour, how many liters of water (per hour) must be vaporized to keep the pipes cool? 2.02 L How many mL of sweat (assume sweat to be the same as water) would cool your body down by 25000 joules? 11 mL Combining specific heat with phase change: How much heat would be required to raise a 200. mL sample of water from 50.0 oC to 125oC? This problem actually has 3 steps: 1. Heat liquid water from 50-100oC No phase change is occurring. We’re just going up in temp by 50oC, so we use q = c x m x ∆T q = (4.18 J/g*oC)200. g(50oC) = 41800 J 2. Boil the water. q = m x Hvap. = 200. g (2260 J/g) = 452000 J 3. Heat the water vapor from 100 – 125oC Once again, no phase change, so… q = c x m x ∆T = (1.87 J/g*oC)200 g(25oC) = 9350 J Finally, add up all 3 answers: 41800 J + 452000 J + 9350 J = 503150 J Step 3 Step 1 Step 2