Physical methods of analysis
advertisement

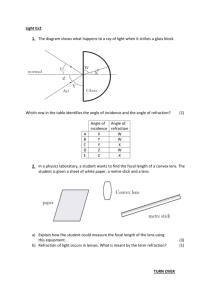
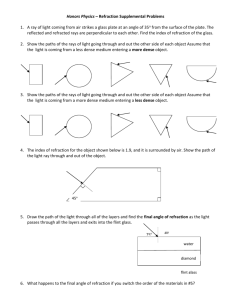
Physical methods of analysis: classification and metrological characteristics Assistant of the pharmaceutical chemistry department Burmas Nataliya Ivanivna e-mail: Natashenka-Burmas@rambler.ru Plan 1. Classification the methods of analysis, advantages and lacks of the physical methods of analysis (PMA). 2. Metrological characteristics of PMA and physical and chemical methods of analysis (PCMA). 3. Refractometry. 4. Polarimetry. 1. Classification the methods of analysis, advantages and lacks of the physical methods of analysis (PMA) Methods of analysis are divided into: 1.Chemical methods: 3. Physical methods: - gravimetry - spectral - titrimetry - kernel-physical 2. Physical and - mas-spectroscopic chemical methods: - thermal - electrochemical 4. biological methods - photometric - kinetic - chromatographic Physical and physical- chemical methods of analysis are based on the using of the relationship between measurable physical properties of substances and qualitative and quantitative composition. Physical methods of analysis based on measuring the effect caused by the interaction of substance with a radiation – the stream of quantum or particles. physical methods of analysis are such methods in which identification of the element based on the properties of its atoms and and nucleus. Physical properties of materials measured by different instruments (tools), so physical and physicalchemical methods are called instrumental. Advantage of PMA 1. High sensitivity - low limit of detection (10-9 mkg) and the definition. 2. The high selectivity. 3. The expressity. 4. The possibility of automation and computerization. 5. The possibility of analysis on the distance. 6. The possibility of analysis without destroying the sample. 7. The possibility of local analysis. Lacks of PМА 1. Error of determination near 5% (in special case to 20%), then 0,01-0,005 % for gravimetry and 0,1-0,05 % for titrimetry. 2. Reproducibility of results in the individual methods is lower than in classical methods of analysis. Lacks of PМА 3.The complexity of the equipment used, its high cost and also high value of standard substances. 4.The need for standards and standard solutions, calibration equipment and construction of calibration graphs. 2. Metrological characteristics of PMA and physical and chemical methods of analysis (PCMA) The sensitivity of method is determined by factors: a) intensity of measured physical properties b) sensitiveness of detectors The little-intensity properties : refraction of light, rotation of the plane polarization of light – refractometry and polarimetry has a low sensitivity. The hight-intensity properties : absorption of light (from nature), fluorescence, radio-activity, emission – are the sensitivity of such methods from 10-6 to 1015 g. Sensitivity of some instrumental methods of analysis Method Limit of exposure Photometry (PMA) 110-6 Fluorimetry (PMA and PCMA) 110-10 Polarography (PCMA) 110-8 Emission spectral analysis (PMA) 110-10 Atomno-absorbcition analysis (PCMA) 110-10 Gas chromatography (PCMA) 110-11 Radioisotope analysis (PMA) 110-15 Mas-spectroscopic (PMA) 110-12 Coulometry (PCMA) 110-10 Kinetic analysis (PMA and PCMA) 110-11 The selectivity of method is the selectivity determination of this substance in presence other without of previous separation High selectivity is inherent the methods, which are based on characteristic properties of molecules, functional groups, atoms, which in same queue own emission and absorbcition properties, radio-activity, ability to electrochemical oxidization or reduction. Low selectivity is characteristic, for example, refractometry is used for the analysis of individual substances or mixtures with 2-3 substances. The regularity of method is closeness the got result to the truth value of content of the determined substance in the experimental object Depends on how measured physical property is adequately reflects the structure and connected with it is strictly defined by laws. Exactness of calibration of analytical equipment will influence on the regularity of method. The standard specimen is a substance which has constant composition and properties. The reproducility of method characterized by variation of results at the parallel measurings. On the producibility influences: the exactness of measuring; the exactness of weighing; the exactness to use of dishes; stability work of the analytical appliance (apply the special methods of measurings). 3. Refractometry Refractometry is a set of methods of the research of physical and chemical properties substance are on the basis of measuring their indexes of refraction by light. Refractometry is a method of qualitative and quantitative analysis, which is based on the measuring the index of refraction by the liquid or crystals. Index of refraction n=sin1/sin 2 Absolute index of refraction (N) is the relation of speed widening of light in the vacuum to its speed in the present medium : N C0 C Relative index of refraction (n) is the relation of speed widening of light in the air to its speed in the present medium: n Cair C Connection between the absolute and relative indexes of refraction is described by a formula: N 1,00027 n The index of refraction is the difficult function which depends from the influence of many factors: a) the nature of refractive medium; b) light is refracted (D =589,3 nm); c) temperature (200,3 С); d) the concentration of solution; e) from direction falling on the crystal of light. In these conditions measuring the refractive index has a value between 1,3-1,7. Exactness of measuring on refraction‘s Abbe arrives from 0,0001 to 0,0003. In practice, refractometry is used to determine the concentration of solution is below 3%. 1. Scale 2. Amici‘s prism (scray of dispersion) 3. Prizmenity block 4. Lighting prism 5. Layer of examinee liquid 6. Measuring prism 7. Crossing for adjusting of light and shade in an eyepiece Qualitative analysis Every clean, optically transparent substance (liquid, crystal) is characterized by the defined numerical value the index of refraction, that used in the qualitative analysis (metanol – 1,3286; ethanol – 1,3613; acetone – 1,3591; chloroform – 1,4456; water – 1,3330). Quantitative analysis It is based on used of dependence between the index of refraction by trial solution and content X of determined substance. n n0 X F n2 n1 F X 2 X1 Basic methods of quantitative analysis: 1. The method of calibration chart n f (X ) 2. Calculation n n0 X F 3. By special of refractometric tables Advantages of the method: simplicity of apparatus design, the speed of execution. Lacks of the method: a low exactness and a low sensitivity (the area determined of contents) X 1% The absolute error doesn't must exceed 0,0002 - 0,005 (for medical means 0,0002) Application: for control quality of substances, solvents and solutions. 4. Polarimetry the method of analysis, which is based on measuring of angle rotation by space polarization of the monochromatic light at passing its through the optically active substance– an individual substance or a solution. has «+» for right-rotation substance has «-» for left-rotation substance The angle rotation of space polarization depends from: a) light b) the thickness of layer c) the nature of a solvent d) the density of optically active medium e) the concentration of optically active substance f) a temperature 20 [ ]D Specific rotation - is the angle rotation of space polarization of monochromatic radiation at thickness of the layer 1 dm and the content of optically active substance 1 g/ml. Specific rotation by the individual substances : [ ] l for solutions of optically active substances: 100 [ ] C l Qualitative analysis it is based on measuring of the angle rotation of space polarization by examinee substance or its solution and calculation of specific rotation. 100 [ ] C l Quantitative analysis calculation method 100 C [ ] l Advantages of method: the simplicity of apparatus design. Lacks of method: a low exactness and a low sensitivity (area of determined contents). Application: a) to control the purity of substances of optically active compounds (carbohydrates, amino acids); b) for conformation of identity and authenticity by the size of specific rotation (ascorbic acid, camphor, levomicetin, morphine); c) for the quantitative determination of sugars (saccharides).