docx
advertisement
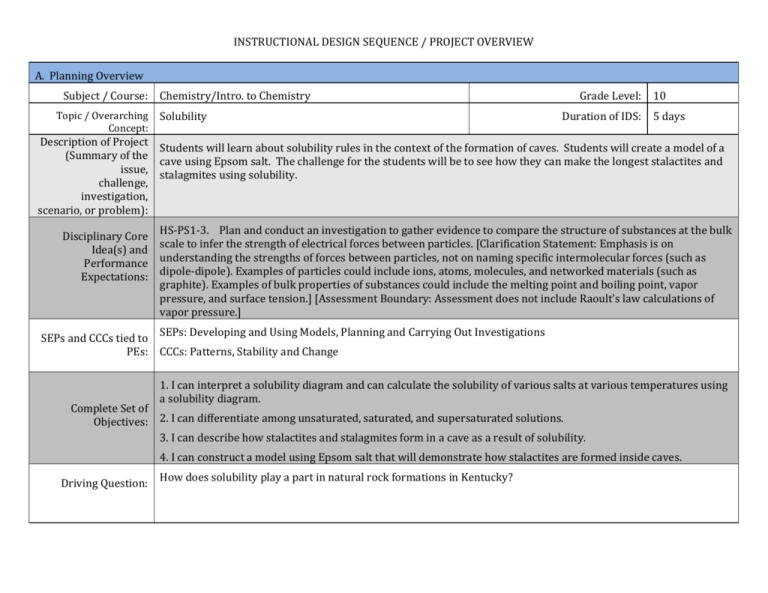
INSTRUCTIONAL DESIGN SEQUENCE / PROJECT OVERVIEW A. Planning Overview Subject / Course: Topic / Overarching Concept: Chemistry/Intro. to Chemistry Solubility Grade Level: Duration of IDS: 10 5 days Description of Project Students will learn about solubility rules in the context of the formation of caves. Students will create a model of a (Summary of the cave using Epsom salt. The challenge for the students will be to see how they can make the longest stalactites and issue, stalagmites using solubility. challenge, investigation, scenario, or problem): Disciplinary Core Idea(s) and Performance Expectations: HS-PS1-3. Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles. [Clarification Statement: Emphasis is on understanding the strengths of forces between particles, not on naming specific intermolecular forces (such as dipole-dipole). Examples of particles could include ions, atoms, molecules, and networked materials (such as graphite). Examples of bulk properties of substances could include the melting point and boiling point, vapor pressure, and surface tension.] [Assessment Boundary: Assessment does not include Raoult’s law calculations of vapor pressure.] SEPs and CCCs tied to SEPs: Developing and Using Models, Planning and Carrying Out Investigations PEs: CCCs: Patterns, Stability and Change 1. I can interpret a solubility diagram and can calculate the solubility of various salts at various temperatures using a solubility diagram. Complete Set of Objectives: 2. I can differentiate among unsaturated, saturated, and supersaturated solutions. 3. I can describe how stalactites and stalagmites form in a cave as a result of solubility. 4. I can construct a model using Epsom salt that will demonstrate how stalactites are formed inside caves. Driving Question: How does solubility play a part in natural rock formations in Kentucky? 1. What is solubility? Other Essential 2. How do temperature and concentration affect solubility? Questions: 3. How does solubility affect cave formation? 4. Why are caves important? 5. How many caves are in Kentucky? 6. How does my model represent what happens in an actual cave? 7. What are the flaws in my model? B. Enactment Overview Entry Event to Launch Students will read an article about the preservation of Mammoth Cave. Students will watch a short 5 minute Project: video about Mammoth Cave. C. Assessment Overview Students know that there are caves in Kentucky, but even though that is the case, students may not have Areas of Conceptual Difficulty or Known Misconceptions Related to visited a cave. Content: Students most likely do not know how caves are formed. It will be important to make sure they know the exact details of this process. As a formal formative assessment, students will be given a target check about reading solubility diagrams, Diagnostic / Formative Assessment: calculating the solubility of salts using the solubility diagrams, and how stalactites form. As an informal formative assessment, I will be monitoring the students during lectures, classwork, and lab work to see how they are progressing with their learning. Summative Assessment: Students will be assessed over this content on a unit exam that will include the other targets from this unit. D. Individual Lessons Plans Day 1: Students will read an article about the preservation of Mammoth Cave. Students will watch a short video about Mammoth Cave. Students will take notes on solubility and solubility curves. Students will practice reading solubility curves and doing calculations with those graphs. Day 2: Students will take notes about cave formation and how it relates to solubility. Students will look at the solubility diagram for Epsom salt and work on determining the exact amount of Epsom salt required for their experiment. Day 3: Students will design their stalactite experiments. Students will be given their supplies and they will have to build their set ups and prepare their solution of Epsom salt based on their calculations. Day 4: Students will set up their stalactite experiments. Day 5: Students will collect data on their stalactite experiments. Students will take a formative assessment over what they have learned about solubility and cave formation. The students will continue to monitor their experiments for one week. Students will be assessed summatively over this target on the unit exam.