Extraction notes
advertisement

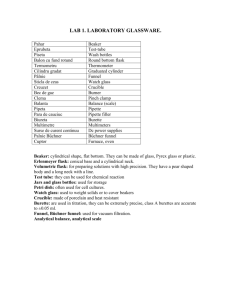
EXTRACTION - a separation technique Extraction • Transferring a solute from one solvent to another • Solvents must be immiscible – usually one is water an one is organic • Solute (the compound being separated) must be more soluble in one solvent than in the other • Usually uses either a separatory funnel or conical vial Separatory Funnel There are many different models! REMEMBER… - Glass to glass requires (a little) grease! - Plastic parts should NEVER be put in the oven! All photos of separatory funnels in this presentation are from the following site: :http://orgchem.colorado.edu/hndbksupport/ext/extprocedure.html Using a Separatory Funnel... 1. Filling – Support funnel. Stopcock must be closed. A clean beaker under funnel – Do not over-fill! Using a Separatory Funnel... 2.Holding Put stopper between index and middle finger -– hold bowl of funnel with thumb and tips of remaining fingers Using a Separatory Funnel... 3. Shaking Make sure stopcock is closed – shake with tip pointed away from everyone – every few seconds, vent the funnel into the hood Using a Separatory Funnel... 4.Layer Identification Usually the more dense solvent is on the bottom – To check, add a few milliliters of distilled water and watch where it goes! Using a Separatory Funnel... 5.Emulsions Emulsions are collodial mixtures of the two solvents. To get rid of these, either: (a) add a few mL of sat. NaCl; (b) filter; (c) add small amount water soluble detergent; (d) apply light vacuum; or (e) change solvent. Using a Separatory Funnel... 5.Removing one layer… Bottom layer is removed via stopcock (1) (1) Top layer is poured out (“decanted”) off the top (2) (2) Partition Coefficient For a solute A, the extracting solvent Sx, and the original solvent So Partition (K) = Coefficient [A] in Sx -----------[A] in So Partition Coefficient (gm A/molar mass A) K = [A] in Sx Liter of Sx ---------- = ------------------[A] in So (gm A/molar mass A) Liter of So Molar mass divides out … Partition Coefficient • When the volume of two solvents is the same (V will cancel out), K is reduced to gm A in Solvent x / gm A in Solvent o • If K> 1 ... Solute mostly in extracting solvent (Sx) • Increasing Volume of Sx, increases gm A removed but not always practical Effect of Multiple Extractions Fn = [ Vo (KVx + Ao) ]n Fn = fraction of original solute remaining in So after n extractions with a constant volume of Sx K = partition coefficient Vx = volume of extracting solvent Vo = volume of original solvent (see page 136-137 of text for derivation/explanation) Effect of Multiple Extractions Example: 1 extraction with 30 mL of ether Fn of 1/16 or 0.0625 3 extractions with 10 mL each of ether (same total volume) Fn of 1/216 or 0.00463 Criteria for Extracting Solvent 1. Will not irreversibly alter the solute and does not react with the other solvent. 2. Immiscible with the original solvent. 3. High K for desired material – selectively remove desired solute. 4. Readily separated from solute after removal.. What affects K? • Relative polarities affect solubility of solute in each solvent. • Reversible chemistry can affect solubility (e.g. protonation/deprotonation). Effect of pH on Solubility HA Add base A- Add acid WHY?? (the salt is more water soluble) Add acid RNH3+ RNH2 Add base Extraction Experiment Dissolve 3 g of 1:1:1 (by mass) mixture of … (a) benzoic acid (b) 2-napthol (c) 1,4-dimethoxybenzene … in 30 mL of diethyl ether OCH3 O pKa ~ 9.5 OH C OH pKa ~ 4.2 2-naphthol benzoic acid OCH3 1,4-dimethoxybenzene Dissolve the 3 g mixture of the above compounds in 30 mL of diethyl ether Add 15 mL of saturated NaHCO3 solution Shake. Separate layers. organ ay e r o us l aque ic lay pH ~ 8-9 er compounds remaining in the organic layer (what are these compounds?) Flask A (what is in Flask A?) Add 10 mL of 1.5 M NaOH solution Shake Separate layers Cautiously acidify with HCl Cool in ice Isolate solid s layer aqueou SOLID FROM FLASK A Flask B (what is in Flask B?) Cautiously acidify with HCl Cool in ice Isolate solid organ ic lay pH ~ 12-13 er Dry organic layer with Na2SO4. Decant ether off drying agent Flask C (what is in Flask C?) Evaporate off ether SOLID FROM FLASK B SOLID FROM FLASK C To Prepare your NB… - 10 Compounds needed in Table of Chemical & Physical Properties: benzoic acid, 2-naphthol, 1,4-dimethoxybenzene, diethyl ether, sodium bicarbonate, sodium hydroxide, sodium chloride, water, concentrated hydrochloric acid, anhydrous sodium sulfate. - Figures needed: - Flowchart (can print from these notes and tape into NB) - Figures 2.60, 2.61, and 2.62 of the text book. - The Handout (posted on Moodle with Flowchart) is your procedure for lab. This can also be printed and taped into your NB, but make sure that you’ve read and understand what you’re going to be doing in lab!!! To Prepare your NB… (cont’d) - There are 4 Chemical Reactions. You need to figure out how to correctly write these AS CHEMICAL STRUCTURES: (1) Benzoic Acid + Na(HCO3) ?? + ?? (2) Sodium benzoate + HCl ?? + ?? (3) 2-naphthol + NaOH ?? + ?? (4) Sodium 2-naphtholate + HCl ?? + ?? To Think About… -What is the definition of a Bronsted-Lowry Acid? - How do you identify an “acidic hydrogen?” (Hint: Why type of atoms are they bonded to before they are pulled off by a base or “fall off” as H+ ?) - pKa is a relative measure of acidity. Acidity increases with decreasing (more negative) pKa values. - Comparing the pKa of a molecule with the pH of the solution that it’s dissolved/mixing in will indicate if the molecule is charged or neutral. - Remember, that when talking about solubility, “like dissolves like.” So polar dissolves in polar, and nonpolar dissolves in nonpolar. - What is the function of anhydrous sodium sulfate??? Complete the Experiment by.. - Submit the samples of all three solutes which you recovered by extraction (properly labeled in a vial!) - Allow the samples to air dry in drawer (for 1 week). - Weigh and record recovered masses of each compound next lab period. (Employ weigh-by-difference technique so pre-weigh empty vials/lid/tape!) - Calculate exactly how much (grams) of each compound was in the original ~3 g sample. - Then, calculate the %-recovery of each compound in Flask A, B, and C. (Remember, the ~3 g original mixture had a 1:1:1 ratio of the three compounds. Ideally, you should have retained about the same amount, b/c just separating.) More info… • How to test acidity of a solution using Blue Litmus Paper: http://www.youtube.com/watch?v=6DCB WK_Hg5w • How to dry over anhydrous sodium sulfate: http://orgchem.colorado.edu/Technique/P rocedures/Drying/Drying.html