CHEMICAL BONDING Set 4
advertisement

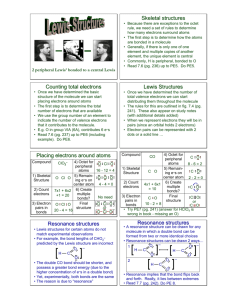
CHEMICAL BONDING Set 4 Cocaine SAVE PAPER AND INK!!! When you print out the notes on PowerPoint, print "Handouts" instead of "Slides" in the print setup. Also, turn off the backgrounds (Tools>Options>Print>UNcheck "Background Printing")! Credits • Thank you to Mr. Neil Rapp who provided the bulk of this powerpoint on his website www.chemistrygeek.co m • Other information comes from Zumdahl, Steven, and Susan Zumdahl. Chemistry. Boston: Houghton Mifflin, 2003. Violations of the Octet Rule Usually occurs with B and elements of higher periods. Common exceptions are: Be, B, P, S, and Xe. Be: 4 B: 6 P: 8 OR 10 S: 8, 10, OR 12 Xe: 8, 10, OR 12 SF4 BF3 Lewis Structures: Comments about the Octet Rule • The second-row elements C, N, O and F should always be assumed to obey the octet rule. • The second-row elements B and Be often have fewer than eight electrons around them in their compounds. These electron-deficient compounds are very reactive. • The second-row elements never exceed the octet rule, since their valence orbitals (2s and 2p) can accommodate only eight electrons. • Third-row and heavier elements often satisfy the octet rule but can exceed the octet rule by using their empty valence d orbitals. • When writing the Lewis structure for a molecule, satisfy the octet rule for the atoms first. If electrons remain after the octet rule has been satisfied, then place them on the elements having available d orbitals (elements in Period 3 or beyond). Resonance • Resonance occurs when more than one valid Lewis structure can be written for a particular molecule. • Example: Nitrate Ion Resonance Explained • If any one of these structures accurately represented the bonding in the nitrate ion, there should be two types of N-O bonds observed in the molecule: one shorter bond (the double bond) and two identical longer ones (the two single bonds). • Experiments clearly show that the nitrate ion exhibits only ONE bond length and bond strength between those expected for a double and single bond. • Resonance structures are the depictions of all possible structures, but in reality, the molecule’s bonding is the average of all three. Structural Isomers http://xsiborganic1011.wikispaces.com/TangYaoFlo • Examine the three models of the three alkanes above to determine how they are similar and how they are different. •All three have 5 carbon atoms and 12 hydrogen atoms, so they all have the molecular formula C5H12. •BUT, you can see that the arrangements of the atoms results in three different compounds: pentane, 2-methyl butane, and 2,2 –dimethyl propane Isomer Definitions • Isomers are two or more compounds that have the same molecular formula, but their atoms are bonded in different arrangements. • Structural isomers have different chemical and physical properties despite having the same chemical formula. • The b.p. of each of the three isomers shown in the previous slide are as follows (in order as shown): 36°C, 28°C, 9°C – This observation supports a main principle of chemistry: structure determines properties. – How does the trend in boiling points of C5H12 isomers relate to their structures? Number of Possible Isomers • As the number of carbons in a hydrocarbon increases, the number of possible structural isomers increases. – There are nine alkanes with the molecular formula C7H16 – There are more than 300,000 structural isomers with the formula C20H42 Odd-Electron Molecules • Relatively few molecules formed from nonmetals contain odd numbers of electrons. • Two common examples are NO and NO2 (try drawing them) • Since the Lewis structure model is based on electron pairs, it does not handle odd-electron cases in a natural way. We usually just tack on the odd electron onto the central atom. Homework Questions • 1) Which has the greater bond lengths: NO2- or NO3- ? Explain. (HINT: You’ll have to draw the Lewis structures first) • 2) The following ions are best described with resonance structures. Draw the resonance structures: a) NCOb) CNO• 3) Explain the terms resonance and its connection to delocalized electrons. Use additional resources if necessary. • 4) Explain the difference between a resonance structure and an isomer.