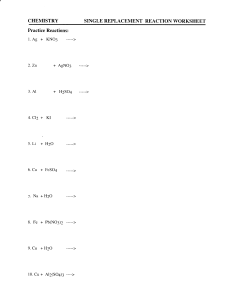
Single Replacement Product Predictions
advertisement

Objective: ◦ Today I will be able to: Determine the purpose of the activity series of metals by completing an online lab Predict the products of single replacement reactions Use the activity series of metals and the periodic table to determine if a single replacement reaction will occur Evaluation/Assessment: ◦ Informal assessment: Listening to student interactions as they complete the lab and practice ◦ Formal assessment: Analyzing student responses to the lab and practice problems Common Core Connection ◦ Use technology and digital media strategically and capably ◦ Build Strong Content Knowledge ◦ Make sense of problem and persevere in solving them Evaluate: Warm – Up Engage and Explore: Single Replacement Online Lab Explain: Single Replacement Reaction Notes Elaborate: Single Replacement Practice Problems Evaluate: Exit Ticket Translate, balance and identify the following reaction ◦ Potassium phospate + hydrogen chloride potassium chloride + hydrogen phospate Today I will be able to: Determine the purpose of the activity series of metals by completing an online lab Predict the products of single replacement reactions Use the activity series of metals and the periodic table to determine if a single replacement reaction will occur Study for Translating, Balancing, Identifying Reactions Quiz ◦ Thursday, February 6 STEM Fair Wednesday, February 5 ◦ (Wear business casual attire) Warm – Up Single Replacement Online Lab Single Replacement Reaction Notes Single Replacement Practice Problems Exit Ticket Complete the lab using the computer. No more that 4 students per group. When you finish, turn in the lab Predicting Products A free element reacts with a compound to form a new compound and to release one of the elements of the original compound General Equation: AB + C AC + B Examples ◦ ◦ 2 HCl + Mg MgCl2 + H2 2 KI + Cl2 2 KCl + I2 Metallic Free •Free element is a metal •Most common •Example •Mg + 2HCl MgCl2 + H2 Non – Metallic Free •Free element is a non-metal •Example •KI + Cl2 KCl + I2 Use the activity series of metals Anything higher on the list as a free element will replace anything lower on the list from a compound Na + PbCl2 2 Na + PbCl2 2 NaCl + Pb This reaction occurs because sodium is higher on the activity series than lead Al + NaCl Al + NaCl No Reaction This reaction does not occur because aluminum is NOT higher on the activity series than sodium Use the periodic table Any free element higher in a family on the Periodic Table can replace any lower element from a compound. Why does this work? Cl2 + NaI Cl2 + 2 NaI 2 NaCl + I2 This reaction occurs because chlorine is higher in its family than iodine S + H2O S + H2O No Reaction This reaction does not occur because sulfur is lower in its family than oxygen Complete the practice at your desk. We will continue to work on the practice problems in class tomorrow. Explain why the reaction between has no reaction ◦ NaF + Br2 No Reaction