Chapter 11 Review Packet
advertisement

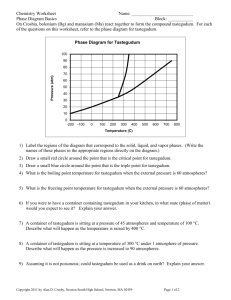
Honors Chemistry Name: ____________________________________ Date: __________________ Mods: __________ Chapter 11 Review A. Directions: On the line at the left, write the term that best matches each description below: vaporization condensation melting point melting/fusion phase change sublimation equilibrium vapor pressure volatile deposition ________________________________ 1) conversion of solid directly into a gas ________________________________ 2) opposite of vaporization ________________________________ 3) temperature at which solid and liquid phases exist in equilibrium. ________________________________ 4) conversion of a substance from one of the three states of matter to another ________________________________ 5) change from a liquid to gas ________________________________ 6) pressure exerted by a constant number of gas molecules in equilibrium with their liquid phase ________________________________ 7) description of a liquid that evaporates easily, with a low boiling point and high vapor pressure ________________________________ 8) transformation of a gas directly into a solid ________________________________ 9) phase change from a solid to a liquid B. Directions: On the line at the left, write the term that best matches each description below: hydrogen bonding ion-dipole forces intermolecular forces london dispersion forces ionic bonding metallic bonding dipole-dipole forces intramolecular forces covalent bonding ________________________________ 9) attractive forces between a polar molecule and an ion ________________________________ 10) attractive forces between molecules near one another ________________________________ 11) attractive forces between nonpolar molecules resulting due to an instantaneous dipole ________________________________ 12) forces within a molecule, responsible for bonding atoms together ________________________________ 13) forces responsible for holding aluminum and chloride ions bound together ________________________________ 14) attractive forces between polar molecules ________________________________ 15) attractive forces between polar molecules when H is attracted to N, O, or F. ________________________________ 16) forces responsible for holding atoms of magnesium bound together C. Directions: Indicate the predominant intermolecular force of attraction between the 2 molecules listed. List of IMFs: ionic bonding metallic bonding ion-dipole forces (IDFs) dipole-dipole forces (DDFs) hydrogen bonding (H-bond) London dispersion forces (LDFs) __________________________________ 1) NaCl and NaCl __________________________________ 2) N2 and N2 __________________________________ 3) H2O and K2O __________________________________ 4) XeF4 and H2 __________________________________ 5) NH3 and NH3 __________________________________ 6) CO2 and O2 __________________________________ 7) Sn and Ni __________________________________ 8) SrCl2 and HBr __________________________________ 9) C5H12 and CH4 __________________________________ 10) PCl3 and CH3Cl __________________________________ 11) Cu and Cu __________________________________ 12) CH3-OH and H2O Use the space below to draw Lewis structures (as needed): D. E. Directions: Rank the following IMFs and intramolecular bonds in order of decreasing strength (with #1 being the strongest, and #6 being the weakest) ________ dipole-dipole forces ________ ionic bonding ________ metallic bonding ________ ion-dipole forces ________ hydrogen bonding ________ London dispersion forces Directions: Each of the following questions consists of two statements, I in the left-hand column and II in the right-hand column. For each question, determine whether statement I is true or false and whether statement II is true or false. Then, fill in the CE oval only if statement II is a correct explanation of the true statement I. Example - True/False: Relationship Analysis Ex (A) I Hydroxide, OH–, is a polyatomic ion Ex (B) Xe is a nonreactive gas BECAUSE II Hydroxide has a net charge and is made up of more than one element Xe has 54 protons in its nucleus. BECAUSE Ex (A) Ex (B) Ex (A): CE is bubbled in because both statements I and II are TRUE and statement I is true BECAUSE of statement II. Ex (B): Even though both statements I and II are TRUE, statement I is not true BECAUSE of statement II (Xe is a nonreactive gas because it has 8 valence electrons, not because it has 54 protons) therefore CE is not bubbled in. *Note: If either statement I or II are false, CE cannot be bubbled in!!!* I II Freezing is an exothermic phase change. BECAUSE 2 London dispersion forces are the strongest of the intermolecular forces. BECAUSE 3 The stronger the IMFs in a substance, the lower the vapor pressure. BECAUSE 4 Water in a glass tube exhibits capillary action. BECAUSE 5 At higher temperatures, a substance has weaker intermolecular attractive forces. 1 BECAUSE 6 The more viscose the substance, the weaker its IMFs. BECAUSE 7 Water has a high surface tension. BECAUSE 1) 5) 2) 6) 3) 7) 4) Heat is released when a gas becomes a liquid London dispersion forces exist due to a brief, instantaneous dipole within a molecule In substances with stronger IMFs, fewer molecules are able to escape to the gas phase, thus reducing the vapor pressure. The cohesive forces between water molecules are stronger than the adhesive forces between water and glass. The greater the kinetic energy of a substance, the further apart and less attracted the molecules are to one another Viscosity is the ease with which a substance evaporates. When interacting with itself, water molecules have very strong cohesive forces. F. Directions: If the statement is true, write “true”. If it is false, change the underlined word or words to make the statement true. Write your answer on the line provided. _________________________________ 1) The average kinetic energy of the particles in a liquid depends upon the temperature. _________________________________ 2) Solids do not flow because the attractive forces between their particles are weaker than those in liquids or gases _________________________________ 3) Dynamic equilibrium is reached when the rate of vaporization exceeds the rate of condensation. _________________________________ 4) Subliming ice is the opposite of freezing water _________________________________ 5) All three states of matter can exist in equilibrium at the triple point G. Directions: Fill in the diagram (with high or low) to show how intermolecular forces influence the volatility, vapor pressure, and boiling point of a substance. weak When IMFs are… strong volatility is ____________ volatility is ____________ vapor pressure is ___________ vapor pressure is ___________ boiling point is ___________ boiling point is ___________ _________________________________________________________________________________ H. Directions: Use the vapor pressure curves below to answer the following questions. 1) What is the vapor pressure of H2O at 50C? ______________________________ 2) What is the boiling point of CCl4 when the external pressure is 30 kPa? ______________________________ 3) What is the normal boiling point of CHCl3? ______________________________ 4) Which substance has the strongest IMFs? ______________________________ I) Directions: Use the heating curve below to answer the following questions. 1) What is the melting point of the substance? __ 2) What is the boiling point of the substance? 3) Which letter represents heating of the solid? 4) Which letter represents heating of the vapor? 5) Which letter represents melting of the solid? 6) Which letter represents boiling of the liquid? J) Directions: Use the phase diagram for water below to answer the following questions. 1) What state of matter is water at 2 atm and 50C ________________________________ 2) What phase change will occur if the temperature is lowered from 80C to -5C at 1 atm? _______________ 3) You have ice at –10C and 1 atm. What could you do in order cause the ice to sublime? _________________________________________________________________________ K) Phase Change Calculations: For problem below involving water, use the following ∆H and Cp values to solve: Melting Point = 0ºC ∆Hfus = 6.00 kJ/mol Cp ice = 2.06 J/g•ºC Boiling Point = 100ºC ∆Hvap = 40.7 kJ/mol Cp water = 4.184 J/g•ºC Cp steam = 1.86 J/g•ºC 1) Given a sample of 145 grams of steam starting at 102.9ºC, determine the quantity of heat (in kJ) released as the sample is cooled down to – 3.7ºC. 2) Calculate the total heat energy, in kJ, needed to convert 100 grams of ice at –13ºC into steam at 105ºC. Is this change endothermic or exothermic? 3) The conversion of 72 grams of steam at 116ºC to liquid at 37ºC releases how many kJ of heat? Is this change endothermic or exothermic? For problem #5 (involving methanol) use the following ∆H and Cp values to solve: Melting Point = –97.6ºC ∆Hfus = 3.18 kJ/mol Cp solid = 84.4 J/g•ºC Boiling Point = 64.7ºC ∆Hvap = 35.28 kJ/mol Cp liquid = 79.5 J/g•ºC Cp gas = 52.3 J/g•ºC 4) How much heat, in kJ, is needed to get 65 grams of solid methanol, CH4O, at –112.6ºC into liquid methanol at 50ºC? Is this change endothermic or exothermic?