Tumor Suppressor Genes and Oncogenes
advertisement

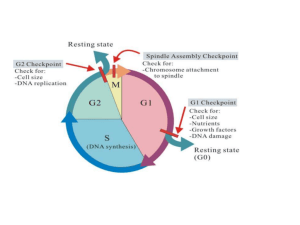
Mechanisms of Mutagenesis & Carcinogenesis Jamil Momand, Ph.D. California State University at Los Angeles Outline (in brief) Introduction Carcinogenesis - a definition Pathways to cancer: overview Oncogenes Tumor suppressor genes Apoptosis Telomerase Angiogenesis Summary Maintenance of homeostasis Adult human maintains ~1015 cells Stem cells undergo ~1012 divisions per day There is a balance between cell birth and cell death Random mutations disrupt homeostasis Molecular basis for cancer progression DNA damage Failure to repair damage Failure to destroy cells with DNA damage Mutation Selection advantage Example of DNA damage From Bertram, Molecular Aspects of Medicine 21, 2001, 161-223 List of carcinogens Chemical Physical Asbestos Gamma radiation Arsenic UV light Chromium Radon Polyaromatic X-rays hydocarbons Viruses* dichlorodiphenyltrichloroethane (DDT) http://www.eur.nl/fgg/ch1/gen_research/dbr-man.html Hereditary form of colon cancer Case 1: Beth M.'s father died of colon cancer, as did her grandmother. Now two of her brothers, both in their 40's, have been diagnosed with colon cancer. Beth, age 37, feels a curse is hanging over her family and is worried about her future and that of her children. Case 2: Paul C. was 35 when his doctor told him the grim news: he had advanced colon cancer. As far as he knew, Paul had no family history of the disease. But after checking, Paul learned that several aunts and uncles had died of colon cancer at an early age. Diagnosis: hereditary nonpolyposis colorectal cancer (HNPCC) Frequency: 1 in 6 colorectal cancer cases Cause of the disease: hMLH1 or hMSH2 mutations. Genes responsible for DNA mismatch repair From Bertram, Molecular Aspects of Medicine 21, 2001, 161-223 Clonal Nature of Cancer Cancers are composed of cells that descended from a single cell. Evidence 1: X-chromosome inactivation Evidence 2: Ig genes in lymphomas and leukemias are identically rearranged. Multistage theory of cancer development (Armitage and Doll, 1954) http://www.hhmi.org/communic/annrep/research/regulate.htm Viruses and cancer Viruses account for 15% of all cancers DNA viruses Epstein-Barr virus Human papilloma virus Hepatitis B virus RNA viruses HIV-1 HTLV-1 HTLV-2 Protection DNA Damage Progression Normal Cell Repair Mutation Cell cycle arrest Proliferation Initiatied Cell Apoptosis Immortality Chemotherapy Radiation therapy Mutator phenotype Cancer Cell Angiogenesis Mutations Proliferation Necrosis Metastasis Adapted from Bertram, Molecular Aspects of Medicine 21, 2001, 161-223 Definitions of terms Amplification Immortal Oncogene Point mutation Proto-oncogene Transformation Translocation DNA Amplification c-myc translocation DNA Methylation and Demethylation-ways to control expression of genes Point mutation activation of Ras Oncoprotein pathways http://www.biocarta.com/pathfiles/pdgfPathway.asp Her2/neu/erbB-2 This gene was discovered by three different groups. That is why it has three different names. Its oncogene counterpart is v-erbB-2 Dr. Slamon (UCLA) described the role of Her2/neu in breast cancer and ovarian cancer. Overexpression, amplification, rare translocations No ligand is known The Philadelphia Chromosome BCR-ABL translocation and expression http://www.kent.k12.wa.us/staff/vhoward/apbio/oncogenes/brc-ablcartoon.gif STI-571--an inhibitor of BCRABL function http://www.blc.arizona.edu/courses/181gh/Lectures_WJG.01/cell_signaling.01/Applications.html PET scanning to show efficacy of STI-571 on tumor metastasis http://www.blc.arizona.edu/courses/181gh/Lectures_WJG.01/cell_signaling.01/Applications.html Design of experiment to clone the first human cellular oncogene (Shi et al., 1979) What we have learned Human cellular oncogenes are dominantacting genes that transform cells and cause tumors in mice. Activation-abnormality that results in higher than normal level of biochemical activity. Point mutation Overexpression Proto-oncogenes-The wild-type noncancerous form of the oncogene How are oncogenes activated? Point mutation-Eg. K-ras, Amplification-Eg. N-myc, MDM2, Her2/neu/ErbB2 Chromosome translocation-Eg. c-myc, bcr-abl Overexpression due to DNA demethylation Tumor suppressor genes •Somatic cell hybrids •Knudsen’s hypothesis •Mutations and mechanisms that lead to tumor suppressor gene loss of function •RB •p53 Somatic cell hybrids Transformed Cell fusion Normal Harris et al., 1969 Normal Genetics of Retinoblastoma Pedigree of Rb-prone family Alfred Knudson Knudson’s hypothesis Mechanisms of tumor suppressor gene inactivation Deletion Point mutation Mutation followed by duplication Loss of heterozygosity DNA methylation Post-translational mechanism-binding to DNA viral oncoproteins Genetic mapping of Rb susceptibility gene RB function http://p53.curie.fr/p53%20site%20version%202.0/p53%20in%20cancer/p53_databaseANAL.html p53 mutation spectrum p53 structure p53 signal transduction pathway [MDM2/p53] complex (transient) degradation p53 Latent p53 Stabilization 1. MDM2 Transcription DNA damage Other p53 functions 2. GADD45 3. WAF1/CIP1 4. 5. } synthesis n DNA Repair Cell cycle arrest Cell Death Functional domains of BRCA1 Transcriptional activation Dimerization Ring finger NLS Proteins that bind BRCA1: BARD1 Rb BAP1 p53 E2F1 Rad51 cMyc Rad50 BRCA1 C-Term. domains BRCA2 p300 RHA RNA PolII CREB binding protein BRCA1 Mapped to chromosome 17q by Mary Clair King in 1990 Linkage was also found in ovarian cancer families. More than 90% of women with germline BRCA1 mutations lose the wild-type allele in breast tumor. The gene encodes a nuclear phosphoprotein of 220 kD (1863 aa’s) The mRNA is 5711 bases long 24 exons, 22 of which is coding BRCA1 (cont. 1) BRCA1-deficient ES cells are hypersensitive to oxidative reagents BRCA1-deficient ES cells are defective in transcription-coupled repair Expression of BRCA1 leads to p21CIP1/WAF1 upregulation and G1-S cell cycle arrest (is this through p53?) BRCA1del11 maintain G1-S cell cycle arrest but not G2-M arrest. http://www.biocarta.com/pathfiles/atmPathway.asp What have we learned about tumor suppressor proteins? Both alleles are deleted in the cancer If one allele is mutated at birth then patients have increased susceptibility to cancer at an early age They often serve as cell cycle checkpoints or DNA repair activities Apoptosis-programmed cell death BCL-2 is a protooncogene that gets overexpressed in some B-cell leukemias Other processes that affect tumor formation and growth Telomerase expression Angiogenesis The BIG picture-a molecular analysis of mutations in colorectal cancers Correlation of tumor morphology to specific allelic deletion (Vogelstein et al., 1988) Future studies-profiling cancers with DNA arrays http://cmgm.stanford.edu/pbrown/ http://www.pnas.org/cgi/content/full/241500798 Dilbert by Scott Adams San Gabriel Valley Tribune, Sept. 10, 2000 References Bertram, J.S. (2001) The molecular biology of cancer. Molecular Aspects of Medicine 21, 167-223. Gelehrter et al. Principles of Medical Genetics, 2nd ed. pp 245272, Williams & Wilkins, Baltimore, 1998. Levine and Lane, (2000) Surfing the p53 network. Nature 408, 307-310 Angier, N., Natural Obsessions: Striving to Unlock the Deepest Secrets of the Cancer Cell, Mariner Books/Houghton Mifflin Co, 1999. Bazell,R., Her-2: The Making of Herceptin, a Revolutionary Treatment for Breast Cancer, Crown Publishing Group, 1998.