DRUG RECEPTOR INTERACTION
advertisement

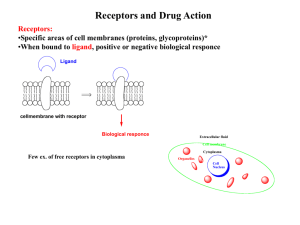
LECTURE 4 PHARMACOLOGY Drug Receptors and Pharmacodynamics The action of a drug on the body, including receptor interactions, dose-response phenomena, and mechanisms of therapeutic and toxic action. Receptors Definitions Classification Ligands (Drugs) Ligand-Receptor interaction Interaction and consequence Receptor-mediated mechanism of action Binding Site Receptor Specific and Saturable Binding Site + Effect Acceptor Binding Site + no Effect DRUG TARGETS many drugs inhibit enzymes Enzymes control a number of metabolic processes A very common mode of action of many drugs in the patient (ACE inhibitors) in microbes (sulfas, penicillins) in cancer cells (5-FU, 6-MP) some drugs bind to: proteins (in patient, or microbes) the genome (cyclophosphamide) microtubules (vincristine) most drugs act (bind) on receptors in or on cells form tight bonds with the ligand exacting requirements (size, shape, stereo specificity) can be agonists (salbutamol), or antagonists (propranolol) receptors have signal transduction methods 1. enzyme linked 2. ion channel linked (multiple actions) (speedy) 3. G protein linked 4. nuclear (gene) linked (amplifier) (long lasting) A macromolecular component of a cell with which a drug interacts to produce a response Usually a protein 1. 2. 3. 4. Regulatory – change the activity of cellular enzymes Enzymes – may be inhibited or activated Transport – e.g. Na+ /K+ ATPase Structural – these form cell parts B. Second Messengers •Small, nonprotein, water-soluble molecules or ions •Readily spread throughout the cell by diffusion •Two most widely used second messengers are: 1. Cycle AMP 2. Calcium ions Ca2+ 2. Calcium Ions (Ca2+) and Inositol Trisphosphate •Calcium more widely used than cAMP •used in neurotransmitters, growth factors, some hormones •Increases in Ca2+ causes many possible responses: •Muscle cell contraction •Secretion of certain substance •Cell division Two benefits of a signal-transduction pathway A. 1. Signal amplification 2. Signal specificity Signal amplification •Proteins persist in active form long enough to process numerous molecules of substrate •Each catalytic step activates more products then in the proceeding steps Receptors and Drug Action Receptors: •Specific areas of cell membranes (proteins, glycoproteins)* •When bound to ligand, positive or negative biological response Ligand cellmembrane with receptor Biological responce Extracellular fluid Cell membrane Few ex. of free receptors in cytoplasm Cytoplasma Organelles Cell Nucleus Drugs that do act on receptors: Drugs that do not act on receptors: Acetylcholin (Neurotransmittor) Antacids: CaCO3 + HCl ca. 5Å Acetylcholin Agonists Diuretics (osmotic) O Pilocarpine Carbacholin N O Me N O Alkylating agents (cancer) O H H2N O N O Cl Cl Cl Cl N Nu N O N H Acetylcholin Antagonists N Nu Atropin N Psoralenes N HN O O N O NH2 R O O R' O N HN h N N OH O O Cyclopentolat O N R O O O R' O O O NH2 N OH O Agonist: Binds to (have affinity for) receptor Binding leads to biolog. response (Agonists have intrinsic activity / efficacy) Antagonist: Affinity for receptor No intrinsic activity Types of receptors-Mechanism of Action Superfamily Endogenous ligands General structures 1 Fast neurotransmitters ex. Acetylcholine Ligand gated ion channels 2 Slow neurotransmitters. ex. noradrenalin G-Protein coupled receptors Hormones 3 Insulin Growth factors Enzyme coupled receptors Catalytic receptors 4 Steroid hormones Thyroid hormones Vitamin A, D Cytoplasmic receptors Ligand gated ion channels Ligand binding sites Ligands Fast neurotransmitters ex. Acetylcholine (nicotinic receptors) Membrane (Phospholipides) Ion chanel Fastest intracellular response, ms Binding of ligand - opening of channel - ion (K+, Na+) in or out of cell - response Nobel prize chemistry 2003, Roderick MacKinnon “for structural and mechanistic studies of ion channels”. http://nobelprize.org/chemistry/laureates/2003/press.html G-Protein coupled receptors G-protein: Guanine nucleotide binding protein Ligand (Agonist) Extracellular fluid Target Intracellular fluid Conform. change Reseptor GDP G-protein GTP +P GDP GDP O HN N N H2N N O O O P O P O O O O n HO OH GTP Responce n=1; GDP n=2; GTP GTP Subtypes of G-proteins - Targets (Second messenger systems) Ion channels: G12 Na+ / H+ exchange Enzymes: Gi Inhib. Adenylyl cyclase Gs Stimul. Adenylyl cyclase Gq Stimul. Phospholipase C One ligand can bind to more than one type of G-protein coupled rec second messenger pathways NH2 NH2 N N Adenylyl cyclase O P O P ATP OH N N N O O O N O P O P O P O O O O O HO N N O O P O O OH c-ATP Activate c-AMP dependent protein kinases (Phosphoryl. of proteins, i.e. enzymes) Various responces (ex. metabolism, cell division) Subtypes of G-proteins - Targets (Second messenger systems) Ion channels: G12 Na+ / H+ exchange Enzymes: Gi Inhib. Adenylyl cyclase Gs Stimul. Adenylyl cyclase Gq Stimul. Phospholipase C Various processes in cell Various processes in cell second messenger pathways Release Ca2+ Activate protein kinases O R R' O O Phopholipase C O O O O P O OH O HO HO R O O P O OH O O + HO HO O P O O OH O O R' O P P DAG Diacylglycerol IP3 Inositol-1,4,5-triphosphate P PIP2 Phosphatidylinositol besphophate Several steps Li (Treatmen manic depression) G protein-linked receptors Structure: •Single polypeptide chain threaded back and forth resulting in 7 transmembrane å helices •There’s a G protein attached to the cytoplasmic side of the membrane (functions as a switch). 2004-2005 Enzyme coupled receptors - Catalytic receptors Ligands: Peptide hormones 1) Binding of Ligand 2) Dimerisation of reseptor Tyr kinase -OH domain (Janus kinase) Phosphoryl of Tyr -OH -OH STATS protein O P O P O P O P STATS protein STAT: Signal transducers and activators of transcription 1) Phosphoryl. of STAT 2) Release of STAT in cytoplasma 3) STATto cell nucleus 4) Intitation of transcription Ion channel receptors Structure: •Protein pores in the plasma membrane Cytoplasmic receptors (not bound to cell membranes) Lipophil. ligand thru cell membrane Cell membrane Cytoplasma Responce Cell nucleus HSP-90 Protein HSP-90 DNA DNA (HSP-90: Heat shock protein) mRNA Intracellular receptors Not all signal receptors are located on the plasma membrane. Some are proteins located in the cytoplasm or nucleus of target cells. • The signal molecule must be able to pass through plasma membrane. Examples: ~Nitric oxide (NO) ~Steroid (e.g., estradiol, progesterone, testosterone) and thyroid hormones of animals). Examples: Glucocorticoids: Inhibit transcription of COX-2; induce transcription of Lipocortin Mineralcorticoids: Regulate expression of proteins involved in renal function Retinoids (Vit A derivatives): Control embryonic development of limbs and organs; affect epidermal differentiation => dermatological use (Acne) PPARs (Peroxisome Proliferation-Activated Receptors): control metabolic processes: PPAR: Target of Fibrates (cholesterol lowering drugs: stimulate oxidation of fatty acids) PPAR: Target of Glitazones (anti-diabetic drugs: induce expression of proteins involved in insulin signaling => improved glucose uptake) Tyrosine-kinase receptors Structure: •Receptors exist as individual polypeptides •Each has an extracellular signal-binding site •An intracellular tail with a number of tyrosines and a single å helix spanning the membrane Receptor subtypes Most receptor classes - several sub-typesDet somatiske nerves ystem Each subtypes - differend A(nta)gonists CNS Sub types cholinerge receptors Acetylcholin (Neurotransmittor) Det autonome nervesys tem Det sympatiske Det paras ympatis ke nervesystem nervesystem CNS CNS ganglion Acetylkolin ca. 5Å Noradrenalin O Synapse O O N Reseptor Effektor celle H Muscarinergic receptors M1: G-Protein coupled receptors Stimulate phospholipase A 4.3 Å HO O O Nicotinerge receptors N Nmuscle: Ligand gated ion channel 5.9 Å M2: G-Protein coupled receptors Inhib. adenylyl cyclase Incr. Na+/Ca2+ N H H N O H Nneuro: Ligand gated ion channels Incr. Na+/Ca2+ Spare receptors - Partial agonist Full agonist Full responce Spare receptors Partial agonist Weak responce Full responce Responce = Agonist Absens of full agonist Responce Presence of full agonist = Antagonist Desensitizing Receptor and normal amount of ligand = agonist Overstimulated receptor Sensitizing Receptor and normal amount of ligand = Antagonist Overstimulated receptor Binding of ligand to receptor •Covalent bond •Ionic bond •Hydrogen bond •Hydrophobic interaction Covalent bond strong; 50-150 kcal/mol, Normally irreversible bonding ex. Acetylcholine esterase inhibitors Acetylkolin-esteras e N O O Kolin Acyltransferas e Acetylkolin (AcCh) Synapse Nerve cell Effector celle reseptor Nerve potensial AcCh AcCh AcCh Ch-acetyltransferase AcCh (exess) Ch AcCh-esterase Ch N Kolin (Ch) OH Reversible inhibitors O AcCh OH O N O O k3(AcCh) OH O OH N HO O OH Inhibitor Me2N O O R O k3(inhibitor) NMe2 OH O Me2N HO OH R More difficult to cleave Reversible inhibitor (drugs): k3 (inhib) < k3(AcCh) Neostigmine Pyridostigmine N Myasthenia gravis (weak muscles, reduced sensitivity to Acetylcholin O O O N N O N Irreversible Inhibitors Not drugs, nerve gasses, insecticides etc. Gen structur mustard gasses L R2 P O R1 N O L: Leaving group R1: alkoksy R2: alkyl, alkoksy, amino N P F O P O O O O O O Sarin Tabun OH O Aging OH R2 P O OR O P R1 O L not tox. Act as mustard gasses Pralidoxim OH HO N R1 N Me Pralidoxim motgift OH OR O P R1 O N N Me O Malaoxon P O R1 OH only insects O P O O S O O Malation OR L O O S P O S P OR O Ionic bond 5-10 kcal/mol, Reversible bonding Hydrogen bond 2-5 kcal/mol, Reversible bonding Hydrophobic interaction 0.5-1 kcal/mol, Reversible bonding Acetylcholin O O O O H N O O N H Anion cavity N Lipophilic area H H-bind acceptor HO O OH The occupancy theory: The more receptors sites occupied by ligand, the stronger response The rate theory: The more ligand-receptor interact / unit time, the stronger response The induced-fit theory: Agonist Receptor Conformation A No ligand bound + Agonist Responce Conformation B + Antagonist Antagonist ConformationA or Inactive conformation C The macromolecular pertubation theory: (induced fit + rate theory) Responce The activation -aggregation theory: Always dynamic equilibrium. Agonist Responce + Agonist Responce Conformation B (Active) Antagonist + Antagonist Responce Inv. Agonist Responce B (opposite of responce A) Responce A + Inverse agonist Dose-Response Relationships [A] - equil. towards AB A-B A + B L-R L + R [L] - equil. towards LR ?? L L-R R locked in membrane (do not move freely) L dissolved in extracellular fluid R Reaction on solid - liquid interface Biological responce Drugs or endogenous compounds binding to receptors are described as Ligands. Ligands are classified into 2 groups Agonist: molecule that binds to receptor and produces similar response to that of the endogenous ligand Partial agonist agonist that produce partial effect Antagonist: molecule that binds to a receptor, but does not cause a response Competitive reversible or weak binding Non-competitive non-reversible or strong binding Ligands (Agonist and Antagonist) Affinity: the attraction of the drug for the receptor. high affinity: low concentrations bind low affinity: high concentrations bind no affinity: does not bind Efficacy: the intrinsic activity Max. effect Min. effect efficacy = 1 efficacy = 0 NEXT TOPIC