The Total Synthesis of Mitomycins
advertisement

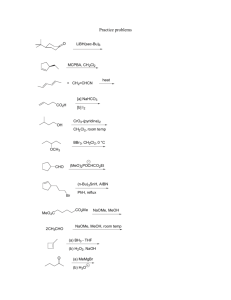
The Total Synthesis of Mitomycins Bob Moreau Organic Supergroup April 25, 2007 O OCONH2 X OMe N Me NH O Mitomycin A X = OMe Mitomycin C X = NH2 Mitomycin A was isolated from the culture broth of Streptomyces caesipitosus in 1956 and mitomycin C in 1958. Their gross structures as well as their relative and absolute stereochemistries were determined by X-ray crystallographic analysis in an effort that took about 20 years. These mitomycins are active against Gram-positive and Gram-negative bacteria, and also show broad activity against tumor cells. Mitomycin C has proven to be more potent and is a widely prescribed antitumor agent. These molecules exert their powerful biological activity by crosslinking DNA strands. Mechanism of Action O H2N OMe reduction NH N Me O OCONH2 H2N OMe N Me O OCONH2 NH -MeOH H2N NH N Me O(H) O(H) O OCONH2 Mitomycin C alkylation of ds DNA O H2N DNA N Me O O DNA oxidation H2N DNA OCONH2 DNA crosslinking H2N O O(H) NH2 DNA DNA N Me NH2 DNA N Me O(H) NH2 Tomasz, M.; Lipman, R.; Chowdary, D.; Pawlak, J.; Verdine, G. L.; Nakanishi, K. Science 1987, 235, 1204. Covalent Crosslink Adduct Molecular model of the Mitomycin C/DNA crosslinked complex showing the mitosene unit snugly fit into the minor grove. Based on this model, the mitosene unit protrudes less than 1 Å beyond the edges of the DNA backbone Tomasz, M.; Lipman, R.; Chowdary, D.; Pawlak, J.; Verdine, G. L.; Nakanishi, K. Science 1987, 235, 1204. Mitomycinoid Structures O X OMe N Me O OCONH2 NH MeO O OMe N NMe O Mitomycin F X = OMe Porfiromycin X = NH2 N O O Isomitomycin A O OCONH2 X X OH N Me OMe NH MeO Me N OCONH2 O Albomitomycin A OCONH2 X Me N O Mitomycin A X = OMe Mitomycin C X = NH2 H OMe Me O OCONH2 NMe O Mitomycin B X = OMe Mitomycin D X = NH2 OR N Me NMe O Mitomycin G X = NH2, R = Me Mitomycin H X = OMe, R = H Mitomycin K X = OMe, R = Me “The complexity of the problem arises from the need to accommodate highly interactive functionality in a rather compact matrix and to orchestrate the chemical progression such as to expose and maintain vulnerable structural elements as the synthesis unfolds. The synthesis of a mitomycin is the chemical equivalent of walking on egg shells.” Danishefsky, S. J.; Scheryantz, J. M. Synlett. 1995, 475. The Kishi Lab Approach O OCONH2 MeO OMe N Me O MeO O MeO OCONH2 X OMe NH NH Me O OCONH2 O Me HN NH Me O HN O Mitomycin A OP PO MeO MeO PO MeO X OMe NH Me OMe Me Me PO PO NH2 Kishi’s Model System O MeO OMe MeO Me Me O HN PO Me OMe MeO MeO Me Me PO HN O O MeO OMe MeO N Me X O O Me O PO X PO NH2 OMe Synthesis of a Key Aromatic Intermediate Cl MeO Cl MeO TiCl4 Me MeO CH2Cl2, 0 °C O H O mCPBA MeO H O MeO CH2Cl2, 0 °C Me Me MeO MeO NaOMe MeOH, 0 °C 98%, 3 steps OH O MeO MeO PhNMe2, reflux Me MeO 96%, 2 steps K2CO3 acetone, reflux Me MeO OH Br MeO Me MeO Synthesis of a Key Aromatic Intermediate OH O MeO HNO3 MeO Me AcOH Me OMe OH MeO Zn AcOH, 0 °C Me O OH BnBr, K2CO3 DME/DMF reflux 67%, 3 steps OBn 1. LDA, CH3CN CN MeO -30 °C MeO O Me OBn NH OBn Ph O OBn OH MeO O 2. CrO3, H2SO4 aq. acetone 71%, 2 steps MeOH/dioxane Me OBn 77% Me OBn Medium Ring Formation OBn OBn MeO CN MeOH, H+ O Me MeO LAH Me OBn OMe OMe OBn OMe OMe OBn NH2 MeO Me NC OBn H2, Pd/C MeOH OMe OMe O MeO Me HN O 40-50%, overall O MeO OMe OMe O2 MeOH Me O NH2 OH OMe OMe OH NH2 MeO Me Transannular Cyclizations OMe OMe O MeO Me H+ in MeOH, SiO2, or heat O O MeO OMe N Me HN O BF3.OEt2 MeSH, -45 °C SMe OMe O MeO Me HN O O HgCl2, Et3N CH2Cl2 N Me O O MeO MeO OMe N Me O Transannular Cyclizations OMe OMe O MeO Me H+ in MeOH, SiO2, or heat O O MeO OMe N Me HN O BF3.OEt2 MeSH, -45 °C SMe OMe O MeO Me HN O O HgCl2, Et3N CH2Cl2 N Me O O MeO MeO OMe N Me O Ketal Formation Problems OBn MeO CN O Me 1. H2CO, NaOMe MeOH, 0 °C 69% (2. BnBr) BnO OR MeO CN O Me OBn BnO ketal formation BnO OH MeO CN MeO OMe Me BnO this compound was obtained in only very low yield 1. BH3 xylene reflux 2. H2O2 BnO MeO CN MeO OMe Me BnO A Solution to the Ketal Formation Problems 1. H2CO, NaOMe BnO MeOH, 0 °C CN MeO 69% OBn MeO O Me 2. Ac2O BnO OAc SMe . CN BF3 2AcOH MeO O Me OBn OAc NH MeS SMe MeSH, -30 °C Me BnO BnO Et3N, MeOH 71% overall BnO OBn MeO BnO CN MeO OMe Me BnO OBn 1. NaOMe CN MeOH/CH2Cl2MeO HgCl2, Et3N MeO MeOH/THF 85%, 3 steps MeS SMe Me BnO 2. BnBr, KH DMF BnO OAc CN MeS SMe Me BnO Sidechain Functionalization BnO OBn MeO MeO OMe Me BnO 1. LDA; PhSeBr CN THF, -78 °C MeO 2. 30% H2O2 EtOAc/THF OBn BnO CN MeO OMe Me BnO DIBAL CH2Cl2, 0 °C OBn MeO H O MeO OMe Me BnO BnO 1. NaBH4 MeOH CH2Cl2, 0 °C 2. Ac2O, py this reaction took over a week to go to completion BnO MeO OBn OMe OMe OH OH Me BnO OAc BnO + MeO OBn OMe OMe OH OH Me BnO OAc 66% overall BnO 3 eq. OsO4 py/THF 87% OBn MeO OAc MeO OMe Me BnO Installation of the Aziridine BnO MeO OBn OMe OMe OH BnO + OH Me BnO MeO OBn OMe OMe steps OH OH Me BnO OAc BnO MeO OBn OMe OMe O 56% or 93% Me BnO OAc OH 1. LiN3, DMF 150 °C 2. Ms2O, py BnO MeO OBn OMe OMe 1. P(OMe)3 reflux N P(OMe)2 Me O BnO NBn2 2. NaH, THF 81%, 2 steps BnO MeO OBn OMe OMe OMs N3 Me BnO NBn2 1. BnNH2 150 °C 2. BnBr, K2CO3 acetone reflux 51% overall BnO MeO OBn OMe OMe OMs N3 Me BnO OMs Advanced Medium Ring Formation BnO MeO OBn OMe OMe BnO LAH N P(OMe)2 O Me BnO MeO Et2O, 0 °C NH Me BnO 90% NBn2 OBn OMe OMe BnO MeI, K2CO3 acetone, reflux MeO OBn OMe OMe NMe Me BnO NBn2 NBn2 H2, Pd/C AcOH OMe OMe O MeO NMe Me HN O OH OH OH OMe OMe O + MeO NMe Me OH O2 MeOH HN O 32%, 3 steps MeO OMe OMe NMe Me OH NH2 Reactivity of the Advanced Medium Ring OH OH OMe OMe O MeO NMe Me OMe OMe O MeO + NMe Me HN O O aq. HCl MeOH OH MeO N Me HN NMe O O O Cl OPh py, 0 °C O MeO OCO2Ph OMe OMe NMe Me HN O O + MeO OCO2Ph OMe OMe NMe Me HN O O MeO CH2Cl2 OPh OMe OMe NMe Me HN O Reactivity of the Advanced Medium Ring OH OH OMe OMe O MeO NMe Me OMe OMe O MeO + NMe Me HN O O aq. HCl MeOH OH MeO N Me HN NMe O O O Cl OPh py, 0 °C O MeO OCO2Ph OMe OMe NMe Me HN O O + MeO OCO2Ph OMe OMe NMe Me HN O O MeO CH2Cl2 OPh OMe OMe NMe Me HN O Failed Conditions from the Model System OH OMe OMe O MeO NMe Me O HBF4 MeO CH2Cl2 OMe x OMe N Me O 90% OCOCl COCl2, PhNMe2 MeO NMe CH2Cl2/PhCH3 N Me HN O O OH NMe O NH3 CH2Cl2 PhCH3 0 °C BF3.OEt2 MeSH, -45 °C 85% OH SMe OMe O MeO NMe Me HN O O H2N OMe N Me O OCONH2 O Porfiromycin NMe NH3 MeOH OCONH2 MeO OMe N Me O NMe Completion of Porfiromycin OH OMe OMe O MeO NMe Me O HBF4 MeO CH2Cl2 OMe x OMe N Me O 90% OCOCl COCl2, PhNMe2 MeO NMe CH2Cl2/PhCH3 N Me HN O O OH NMe O NH3 CH2Cl2 PhCH3 0 °C BF3.OEt2 MeSH, -45 °C 85% OH SMe OMe O MeO NMe Me HN O O H2N OMe N Me O OCONH2 O Porfiromycin NMe NH3 MeOH OCONH2 MeO OMe N Me O NMe Completion of Mitomycin A BnO MeO OBn OMe OMe NH Me BnO NBn2 1. acrolein CH2Cl2 2. BH3 THF/CH2Cl2 -78 °C to rt 3. Ac2O, py BnO MeO OBn OMe OMe NP Me BnO 78% NBn2 OH 1. H2, Pd/C AcOH 2. O2, MeOH OMe OMe O MeO NP Me 42% HN O HBF4 CH2Cl2 P = (CH2)3OAc 77% 1. NaOMe MeOH/CH2Cl2 OCONH2 2. DMSO, DCC OMe TFA/py O MeO N Me NH O Mitomycin A O MeO 3. HClO4, PhNMe2 Me CH2Cl2 35% OCONH2 OMe N O NP 1. COCl2, PhNMe2 MeO CH2Cl2 2. NH3 CH2Cl2, 0 °C O OH OMe N Me O 85% Nakatsubo, F.; Fukuyama, T.; Kishi, Y. J. Am. Chem. Soc. 1977, 99, 8116. Fukuyama, T.; Nakatsubo, F.; Cocuzza, A. J.; Kishi, Y Tetrahedron Lett. 1977, 49, 4295. Kishi, Y. J. Nat. Prod. 1979, 42, 549. NP The Fukuyama Lab Approach O O OCONH2 H2N OMe N Me MeO NH O MeO OCONH2 H OMe NH N O Mitomycin C H OMe Me O OCONH2 Me N N O Albomitomycin A Isomitomycin A Ph MeO BnO MeO OTMS SEt O O H Me MeO Me N3 MeO Ph BnO MeO Me OTMS SEt O O H N MeO Intramolecular [3 +2] Cycloaddition Ph MeO 13 steps Me BnO EtS Ph O OTMS O MeO MeO 64% overall MeO BnO Me N3 MeO SnCl4 CH2Cl2, -78 °C; then py OTMS SEt O O H Me N3 MeO 95%, >20:1 dr stereoselectivity likely due to an endo Diels-Alder toluene 110 °C Ph BnO MeO Me OTMS SEt O O H N MeO Ph BnO MeO OTMS SEt O O H 86% extrusion of N2 Me N MeO H N N a triazoline Hydroxymethylene Installation Ph BnO MeO OTMS SEt O O H Me Ph N 1. DIBAL THF, -78 °C BnO MeO 2. Ac2O, py 99%, 2 steps MeO OTMS SEt O OAc H Me N MeO RuO2, NaIO4 1:1 EtOAc/H2O 84% O O BnO MeO N H SO2Et O H O CCl3 OAc Me N MeO 1. NaBH4, MeOH 97% 2. O Me . CH2Cl2 MeO O H SO2Et O Ozonolysis gave a complex mixture OAc O N BnO H CCl3 N MeO Unveiling the Pyrrolidine O O O O BnO MeO N H SO2Et O H CCl3 O NH3 O MeO NH2 H O H MeOH, rt O Me BnO N Me :NH3 MeO O N MeO NH3, -H2O O O BnO MeO H O NH2 NaBH4 OH NH Me N MeO O BnO MeO H OH NH 61% overall the bridgehead hemiaminal resisted reduction by NaBH4 NH2 OMe Me N MeO Completion of Isomitomycin A BnO MeO OCONH2 H OH NH Me BnO CSA MeOH, rt N MeO OCONH2 H Me MeO H N N MeO 60% O MeO Me OCONH2 H OMe NH N 1. H2 (1 atm) 10% Pd/C EtOH 2. DDQ, H2O acetone -78 °C O Isomitomycin A BnO MeO Me H OMe NH N MeO 77%, 2 steps OCONH2 Completion of Mitomycin C O MeO Me OCONH2 H OMe NH O NH3 MeOH, rt N O H2N OCONH2 H Me OMe NH N O 85% Al(OiPr)3 MeOH, rt Michael addition 91% O H2N OMe N Me O OCONH2 O NH -elimination H2N OCONH2 H OMe N Me O N Mitomycin C Fukuyama, T.; Yang, L. J. Am. Chem. Soc. 1987, 109, 7881. Fukuyama, T.; Yang, L. J. Am. Chem. Soc. 1989, 111, 8303. Completion of Mitomycin A O MeO Me OCONH2 H OMe NH O NH3 MeOH, rt N O H2N OCONH2 H Me OMe NH N O 85% Al(OiPr)3 MeOH, rt Michael addition 91% O MeO OMe N Me O OCONH2 O NH -elimination H2N OCONH2 H OMe N Me O N Mitomycin A Fukuyama, T.; Yang, L. J. Am. Chem. Soc. 1987, 109, 7881. Fukuyama, T.; Yang, L. J. Am. Chem. Soc. 1989, 111, 8303. Danishefsky’s Approach to FR-900482 OCONH2 OH MeO OH H N O MeO NH N O MeO internal Heck arylation OMe NR MeO O O OMe I N NR O O FR-900482 MeO OMe I O MeO N O + intermolecular hetero Diels-Alder OH MeO OMe I HO MeO N O O An Intramolecular Approach to FR-900482 OCONH2 OH MeO O MeO O N OMe OMe OH H O MeO NH O N MeO O O N O O bridged mode FR-900482 x MeO HO OMe MeO O OMe MeO O h366 nm MeO NO2 O MeOH OMe MeO N O O MeO O N O fused mode Inspiration for a Mitomycin Synthesis OCONH2 OH MeO O MeO O N OMe OMe OH H O MeO NH O N MeO O O N O O bridged mode FR-900482 x MeO HO OMe MeO O OMe MeO O h366 nm MeO NO2 O MeOH OMe MeO N O O MeO O N O fused mode The Danishefsky Lab Approach O MeO MeO MeO OMe N Me NMe N O MeO OMe Me O MeO O NMe OMe N Me MeO MeO O Mitomycin K MeO HO MeO MeO Me MeO Me NO2 MeO OMe intramolecular hetero Diels-Alder MeO O MeO OMe Me MeO N O Hetero Diels-Alder OMe MeO MeO steps Me MeO Li O MeO MeO HO MeO H Me OMe THF, -78 °C NO2 MeO Me NO2 MeO 80% h, 350 nm MeOH h MeO O MeO MeO OMe N Me MeO HO 45% O MeO OMe + Me MeO 15% N O A Sequential Photolytic Redox Mechanism MeO h350 nm Me NO2 OMe MeO HO OMe MeO HO MeOH MeO N MeO MeO 1,5 H abstr. H Me MeO O -H2O OMe O MeO N Me O MeO O [4 + 2] MeO MeO O MeO OMe N Me MeO 1,5 H abstr. MeO OMe Me MeO HO O MeO N O h MeO OMe Me H O MeO N O Aziridine Fragmentation MeO MeO O MeO OMe N Me MeO OMe PDC CH2Cl2 N Me MeO MeO O N Me MeO 85% O 65% BnN3 PhH, 80 °C MeO HO O MeO OMe Bn N N N O 1. h, 254 nm 76% 2. L-Selectride THF, -78 °C 81% MeO MeO MeO O MeO OMe NHBn N Me MeO AIBN Bu3SnH PhH, 80 °C S O OMe Im MeO Im O MeO N Me NBn CH2Cl2 MeO S S Im OMe DMAP N Me MeO 66% HO NBn Synthesis of a Deoxygenation Precursor MeO MeO O MeO OMe N Me MeO O MeO OMe PDC PhS N3 MeO SPh OMe N N Me CH2Cl2 MeO PhH, 80 °C N Me MeO HO O N N MeO O 65% O 90% L-Selectride THF, -78 °C 77% MeO S O MeO SPh OMe N N N MeO O S Im MeO Im O MeO N Me Im DMAP CH2Cl2, 35 °C N N Me MeO 65% SPh OMe N N HO A Successful Deoxygenation MeO O MeO SPh OMe N N Me MeO N N O Im MeO AIBN Bu3SnH MeO PhH, 80 °C MeO O SPh OMe N N MeO S 13% h, 254 nm PhH extrusion of N2 48% MeO MeO O MeO OMe N Me MeO N Me N MeO 52% OMe NH2 + N Me O MeO Raney Ni NMe acetone, 60 °C 70% O MeO OMe N Me MeO N SPh Completion of Mitomycin K MeO TMS O MeO OMe N Me NMe MeO TMS MeO Li THF, -10 °C N Me 90% NMe MeO O NaOAc MeCN/H2O N O O Ag O 8-16% TMS O O MeO OMe N Me OH OMe MeO O NMe PPTS CH2Cl2 81% OH OMe MeO N Me NMe O Mitomycin K Benbow, J. W.; Schulte, G. K.; Danishefsky, S. J. Angew. Chem. Int. Ed. Engl. 1992, 31, 915. Benbow, J. W.; McClure, K. F.; Danishefsky, S. J. J. Am. Chem. Soc. 1993, 115, 12305. Danishefsky, S. J.; Scheryantz, J. M. Synlett. 1995, 475.