Macromolecules - Crestwood Local Schools
advertisement

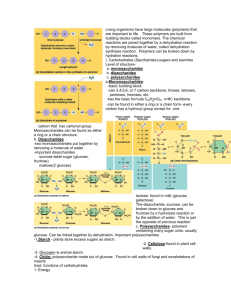
Focus on: Elements in each molecule How molecules are linked and unlinked Examples and functions of each type of molecule Chapter 3 The Chemical Building Blocks of Life Macromolecules Large molecules formed by joining many subunits together. Also known as “polymers”. Monomer A building block of a polymer. Condensation Synthesis or Dehydration Synthesis The chemical reaction that joins monomers into polymers. Covalent bonds are formed by the removal of a water molecule between the monomers. Hydrolysis Reverse of condensation synthesis. Hydro- water Lysis - to split Breaks polymers into monomers by adding water. Four Main Types Of Macromolecules Carbohydrates Lipids Protein Nucleic acids For each Macromolecule know the following: Elements it contains Monomer units and structures Examples Uses or roles Carbohydrates Used for fuel, building materials, and receptors. Made of C,H,O General formula is CH2O C:O ratio is 1:1 Types Of Carbohydrates Monosaccharides Disaccharides Oligosaccharides Polysaccharides Monosaccharides Mono - single Saccharide - sugar Simple sugars. 3 to 7 carbons. Can be in linear or ring forms. Monosaccharides Can be “Aldoses” or “Ketoses” depending on the location of the carbonyl group. Examples Glucose Galactose Ribose Fructose - OSE Word ending common for many carbohydrates. Disaccharides Sugar formed by joining two monosaccharides through a “glycosidic linkage”. Examples Maltose = glucose + glucose Lactose = glucose + galactose Sucrose = glucose + fructose Oligosaccharides 2 - 10 joined simple sugars. Used in cell membranes. Polysaccharides Many joined simple sugars. Used for storage or structure. Examples: Starch Cellulose Glycogen a glucose and b glucose Starch Made of 1-4 linkages of a glucose. Linkage makes the molecule form a helix. Fuel storage in plants. a glucose Cellulose Made of 1-4 linkages of b glucose. Linkage makes the molecule form a straight line. Used for structure in plant cell walls. b glucose Comment Most organisms can digest starch (1- 4 a linkage), but very few can digest cellulose (1- 4 b linkage). Another example of the link between structure and function. Glycogen “Animal starch” Similar to starch, but has more 1-6 linkages or branches. Found in the liver and muscle cells. Starch Glycogen Lipids Diverse hydrophobic molecules. Made of C,H,O No general formula. C:O ratio is very high in C. Fats and Oils Fats - solid at room temperature. Oils - liquid at room temperature. Fats and Oils Made of two kinds of smaller molecules. Fatty Acids Glycerol Fatty Acids A long carbon chain (12-18 C) with a -COOH (acid) on one end and a -CH3 (fat) at the other. Acid Fat Neutral Fats or Triacylglycerols Three fatty acids joined to one glycerol. Joined by an “ester” linkage between the -COOH of the fatty acid and the -OH of the alcohol. Saturated Fats Unsaturated Fats Saturated - no double bonds. Unsaturated - one or more C=C bonds. Can accept more Hydrogens. Double bonds cause “kinks” in the molecule’s shape. Question Why do fats usually contain saturated fatty acids and oils usually contain unsaturated fatty acids? The double bond pushes the molecules apart, lowering the density, which lowers the melting point. Fats Differ in which fatty acids are used. Used for energy storage, cushions for organs, insulation. Question ? Which has more energy, a kg of fat or a kg of starch? Fat - there are more C-H bonds which provide more energy per mass. Phospholipids Similar to fats, but have only two fatty acids. The third -OH of glycerol is joined to a phosphate containing molecule. Result Phospholipids have a hydrophobic tail, but a hydrophilic head. Self-assembles into micells or bilayers, an important part of cell membranes. Steroids Lipids with four fused rings. Differ in the functional groups attached to the rings. Examples: cholesterol sex hormones Proteins The molecular tools of the cell. Made of C,H,O,N, and sometimes S. No general formula. Uses Of Proteins Structure Enzymes Antibodies Transport Movement Receptors Hormones Proteins Polypeptide chains of Amino Acids linked by peptide bonds. Amino Acids All have a Carbon with four attachments: -COOH (acid) -NH2 (amine) -H -R (some other side group) R groups 20 different kinds: Nonpolar - 9 AA Polar - 6 AA Electrically Charged Acidic - 2 AA Basic - 3 AA Amino Acids Amino Acids R groups Contain the S when present in a protein. Cysteine or Cys Methionine or Met The properties of the R groups determine the properties of the protein. Polypeptide Chains Formed by dehydration synthesis between the carboxyl group of one AA and the amino group of the second AA. Produce an backbone of: (N-C-C)X Levels Of Protein Structure Organizing the polypeptide into its 3-D functional shape. Primary Secondary Tertiary Quaternary Primary Sequence of amino acids in the polypeptide chain. Many different sequences are possible with 20 AAs. Secondary 3-D structure formed by hydrogen bonding between parts of the peptide backbone. Two main secondary structures: a helix pleated sheets Tertiary Bonding between the R groups. Examples: hydrophobic interactions ionic bonding Disulfide bridges (covalent bond) Quaternary When two or more polypeptides unite to form a functional protein. Example: hemoglobin Is Protein Structure Important? Denaturing Of A Protein Events that cause a protein to lose structure (and function). Example: pH shifts high salt concentrations heat Comment Many other amino acids are possible (change the R group) Whole new group of proteins with new properties can be made Genetic engineering can use bacteria to make these new proteins Nucleic Acids Informational polymers Made of C,H,O,N and P No general formula Examples: DNA and RNA Nucleic Acids Polymers of nucleotides Nucleotides have three parts: nitrogenous base pentose sugar phosphate Nitrogenous Bases Rings of C and N The N atoms tend to take up H+ (base). Two types: Pyrimidines (single ring) Purines (double rings) Pentose Sugar 5-C sugar Ribose - RNA Deoxyribose – DNA RNA and DNA differ in a – OH group on the 2nd carbon. Nucleosides and Nucleotides Nucleoside = base + sugar Nucleotide = base + sugar + Pi DNA Deoxyribonucleic Makes Acid. up genes. Genetic information for life. RNA Ribonucleic Acid. Structure and protein synthesis. Genetic information for a few viruses only. DNA and RNA More will be said about DNA and RNA in future lessons. Summary Role of hydrolysis and dehydration synthesis For each macromolecule, know the following: Elements and monomers Structures Functions