2) Exp. 1 Report Sheet
advertisement

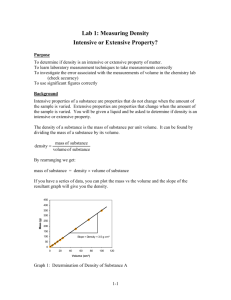
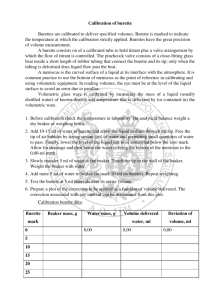
Experiment 1: Lab Equipment Use Fill in your answers as they correspond to the instructions for this lab. In section 1, fill in the data tables collected during lab. In section 2, copy your data and perform your calculations. In section 3, copy your results from the calculations section. Then, answer all the post-lab questions in section 4. SECTION 1: DATA TABLES PART IA: CENTIGRAM BALANCE (1) Precision of Balance (Number of places after decimal point) Direct (using tare) Indirect (by difference) Direct (using tare) Indirect (by difference) (2) Mass of Metal Slug (direct method) (3 & 4) Mass of Beaker or Paper (3 & 4) Mass of Beaker or Paper + Metal Slug PART IB: ANALYTICAL BALANCE (1) Precision of Balance (Number of places after decimal point) (2) Mass of Metal Slug (direct method) (3) Mass of Beaker or Paper (3) Mass of Beaker or Paper + Metal Slug PART IC: MASS OF CHEMICALS (DIRECT METHOD) (4) Mass of Salt Alscher Page 1 of 10 (Modified August 29, 2013 by Isabella Germek), 3/15/2016 PART IIA: WATER TEMPERATURE & DENSITY (IIa-(2)) Temperature of Water CRC Density of Water at the measured Temperature PART IIB: VOLUME MEASUREMENTS; GRAD. CYLINDER (2) Precision on Graduated Cylinder (Number of places after decimal point) (3) Mass of Empty Graduated Cylinder (4) Volume of Sample 1 (eye-level reading) (VR−1 ) (5) Volume of Sample 1 (eye above meniscus) (5) Volume of Sample 1 (eye below meniscus) (6) Mass of Cylinder + Sample 1 (VM−1 ) (8) Volume in Cylinder After Adding Sample 2 (VR−T ) (9) Mass of Water + Cylinder After Adding Sample 1 & 2 (VM−T ) PART IC: MENISCUS READER No data table necessary for Part IC. PART IIC: VOLUME MEASUREMENTS; BURETTE (2) Precision on Burette (Number of places after decimal point) (5) Initial Volume on Burette (VRb−initial )(could be z(could be 0/00mL)(could Could (6) Mass of Empty Beaker (7) Volume of Sample 1 (read on burette) (8) Mass of Beaker + Sample 1 (VRnB−1 ) (9&10) Total Volume Delivered (Sample 1 + Sample 2 Alscher Page 2 of 10 (Modified August 29, 2013 by Isabella Germek), 3/15/2016 are now in beaker) (VRb−T ) (11) Mass of Beaker + Water (Both Samples) (VMb−T ) SECTION 2: CALCULATIONS Use the data from your data tables in section 1 to perform your calculations in this section. Show your calculations using correct significant figures and units. PART IA: CENTIGRAM BALANCE Indirect (by difference) (Ia-4) Copy Mass of Slug and Beaker or Paper (1a-4) Copy Mass of Beaker or Paper Calculate Mass of Slug by Difference PART IB: ANALYTICAL BALANCE Indirect (by difference) (Ib-3) Copy Mass of Slug and Beaker or Paper (Ib-3) Copy Mass of Beaker or Paper Calculate Mass of Slug by Difference PART IC: MASS OF CHEMICALS (DIRECT METHOD) No calculations in Part IC. PART IIB: VOLUME MEASUREMENTS; GRAD. CYLINDER (IIb-5) Copy Volume in Cylinder After Adding Sample 2 (VR−T ) V total (IIb-4) Copy Volume of Sample 1-use the eye-level readings for all calculations. (Read volume of sample 1) VR1 (𝐕𝐕−𝐕 )(𝐕𝐕𝐕𝐕 𝐕𝐕𝐕𝐕𝐕𝐕 𝐕𝐕𝐕𝐕 Alscher Page 3 of 10 (Modified August 29, 2013 by Isabella Germek), 3/15/2016 Calculate Volume of Sample 2 by difference. (read volume of sample 2) VR2. (IIb-3 & 6) Calculate Mass of Sample 1 by difference. (Mass of cylinder with sample 1 less the mass of empty cylinder) M1 (IIb-6 & 9) Calculate Mass of Sample 2, by difference. (Mass of cylinder with sample 1 & 2 less the mass of cylinder with sample 1) M2 Calculate Volume of Sample 1 based on its mass (VM−1 ), and its density from the CRC. Vm-1 Calculate Volume of Sample 2 based on its mass (VM−2 ) and its density from the CRC. Vm -2. Calculate the difference between the read volume and the calculated volume based on mass and density for Sample 1. |(VM−1 ) − (VR−1 )|,ΔV1 (|VR1 + Vm-1|) = ∆V1 Calculate the difference between the read volume and the calculated volume based on mass and density for Sample 2. |(VM−2 ) − (VR−2 )|,ΔV2 (|VR2 + Vm-2|) = ∆V2 Calculate the Average. Use the two changes in volume to calculate the average change in volume. (|∆V1 + ∆V2|)/2 Calculate the average volume based on reading. [Use the volumes of the samples from the calculations section above (VR−1 and VR−2 )to get this average.] (|VR1 + VR2|)/2 Calculate the Average % Difference (see instructions). PART IIC: VOLUME MEASUREMENTS; BURETTE Copy the Total Volume in Beaker after adding both samples. (VRb−T ) Alscher Page 4 of 10 V total (Modified August 29, 2013 by Isabella Germek), 3/15/2016 Calculate the Volume of Sample 1 in the beaker by finding the difference between the initial volume on the burette & volume in the burette after the first delivery. (VRb−1 ) − (VRb−initial ) VR1 Calculate the Volume of Sample 2 in the beaker by finding the difference between the final volume read on the burette, & the volume read on the burette after the first delivery. (VRb−2 ) − (VRb−1 ) VR2. Copy the Mass of the Beaker + Water (Both Samples). (VMb−T ) Calculate the Mass of Sample 1 in the beaker by finding the difference between the mass of Sample 1 with the beaker & the mass of the empty beaker. (VMb−1 ) M1 Calculate the Mass of Sample 2 by finding the difference between the mass of the beaker with both samples and the mass of the beaker with Sample 1. (VMb−2 ) M2 Calculate the Volume of Sample 1 using its calculated mass above (VMb−1 ) and the density from the CRC. Vm-1 Calculate the Volume of Sample 2 using its calculated mass above (VMb−1 ) and the density from the CRC. Vm -2. Calculate the difference between the volume by reading and the volume by mass/density for Sample 1. |(VM−1 ) − (VR−1 )|ΔV1 (|VR1 + Vm-1|) = ∆V1 Calculate the difference between the volume by reading and the volume by mass/density (|VR2 + Vm-2|) = ∆V2 Alscher Page 5 of 10 (Modified August 29, 2013 by Isabella Germek), 3/15/2016 for Sample 2. |(VM−2 ) − (VR−2 )|ΔV2 Calculate the Average Δ𝐕using the two changes in volume calculated above. (|∆V1 + ∆V2|)/2 Calculate the average volume based on reading only using the volumes from reading in the calculations section above. (VRb−1 ) and (VRb−2 ) (|VR1 + VR2|)/2 Calculate the Average % Difference (see instructions). SECTION 3: RESULTS TABLES This section is to organize and consolidate the results of the experiment. Copy your results from the data tables and calculations section. PART IA: MASS MEASUREMENT - CENTIGRAM BALANCE Mass of Slug by Direct Method Mass of Slug by Difference PART IB: MASS MEASUREMENT - ANALYTICAL BALANCE Mass of Slug by Direct Method Mass of Slug by Difference PART IC: MASS MEASUREMENTS USING CHEMICALS Mass of NaCl on Centigram Balance PART IIB: VOLUME MEASUREMENTS - GRAD. CYLINDER Average % Difference= PART IIC: VOLUME MEASUREMENTS - BURETTE Average % Difference= Alscher Page 6 of 10 (Modified August 29, 2013 by Isabella Germek), 3/15/2016 PART III: COST OF LAB DRAWER The Sum of the Costs of the Glassware and Instruments in Your Lab Drawer SECTION 4: QUESTIONS Answer the following questions with complete sentences and good grammar on this report sheet. Alternatively, you can type your answers if you so desire. 1) What is a meniscus? 2) Where is a liquid volume reading taken in relation to a meniscus? 3) In Part IIB, you determined the volume of water based on reading the volume in a graduated cylinder and on the mass of the water in the cylinder. Answer the following questions: a) Were the volumes the same? If they differed, how much did they differ by? For example, your volume by reading might be 10.3 mL and your volume by mass might be 10.8 mL. b) What does the percent difference tell you in terms of your precision using the graduated cylinder? c) Why was it important to take the temperature of the water? 4) Which of the following pieces of equipment is designed to deliver a volume of liquid? Which is designed to contain a specific volume of liquid? Explain your choices a) graduated cylinder, b) pipette, c) burette. Alscher Page 7 of 10 (Modified August 29, 2013 by Isabella Germek), 3/15/2016 5) Suppose a 10-mL burette is used that has calibration marks representing each 1-mL and 0.1mL. Accurate readings should be estimated and recorded to the nearest (a) 1.0-mL, (b) 0.1mL, (c)0.01-mL, (d) 0.001-mL. Choose one and explain your answer. 6) Although we did not use measurement in this lab, we often have to consider significant figures in other tools. Consider a block with rectangular sides whose dimensions have been measured with a millimeter ruler. The height is 254.7 mm, the width is 136.8 mm, and the depth: 25.3 mm. a) Determine the volume of the block in cubic millimeters. b) Determine the volume of the block if you assume that each of the above measurements should actually be 0.1 mm higher than listed. c) With which digit do the answers in parts ‘a’ and ‘b’ begin to differ? If you report the volume using all of the digits that are the same in parts ‘a’ and ‘b’ plus one more digit where the two values do not agree, how many digits should you report? d) How many digits would you report according to the rules for significant figures? Is this the same as in part c? Alscher Page 8 of 10 (Modified August 29, 2013 by Isabella Germek), 3/15/2016 7) The density of water is very close to 1 g/mL at room temperature. You wish to determine the density of a sample with an approximate volume of 25 mL, and an approximate mass of 25 g. To how many significant figures should you report the density if you use the following tools: (Precisions for the tools are listed in Tables 1 & 2 in the instructions.) Please explain your choice in a complete sentence. Example: A top-loading semi-micro balance and a 50-mL burette. For example, the mass of my sample is 25.0000 g using a semi-micro balance. The volume of the sample is 25.00 mL. Since my mass has 6 significant figures and the volume has 4 significant figures, I can report only 4 significant figures. The density would be 1.000 g/mL. a) An analytical balance and 25-mL pipette b) Centigram balance and 25-mL pipette c) Analytical balance and 50-mL burette d) Centigram balance and 50-mL burette 8) A student fills a burette with distilled water, adjusts the meniscus to reread 0.00-mL, and allows water to drain out until a reading of 10.00-mL is obtained. The water sample, weighed by difference has a mass of 9.72 g. Which of the following experimental errors will account for the large difference between the volume according to readings and the volume according to mass? (There might be more than one correct or wrong answer) a)Initially the meniscus was above the 0.00-mL mark. b)The final meniscus was below the 10.00-mL mark. c)Air was not cleared from the burette tip before delivering the sample. Alscher Page 9 of 10 (Modified August 29, 2013 by Isabella Germek), 3/15/2016 a. Alscher d)Water leaked from the burette into the beaker after the final burette reading was taken. Explain your answer. Page 10 of 10 (Modified August 29, 2013 by Isabella Germek), 3/15/2016