Chapter 27: Reproductive System
advertisement

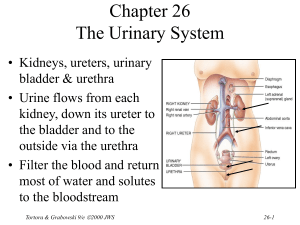
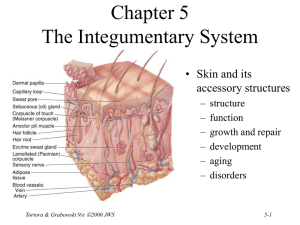
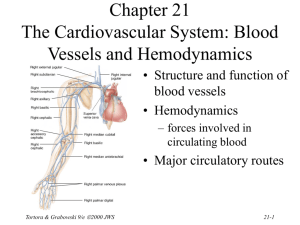
Chapter 27 Fluid, Electrolyte and Acid-Base Homeostasis • Body fluid – all the water and dissolved solutes in the body’s fluid compartments • Mechanisms regulate – total volume – distribution – concentration of solutes and pH Tortora & Grabowski 9/e 2000 JWS 27-1 Balance Between Fluid Compartments Volume of fluid in each is kept constant. Since water follows electrolytes, they must be in balance as well • Only 2 places for exchange between compartments: – cell membranes separate intracellular from interstitial fluid. – only in capillaries are walls thin enough for exchange between plasma and interstitial Tortora & Grabowski 9/e 2000 JWS fluids 27-2 Body Water Gain and Loss • 45-75% body weight – declines with age since fat contains almost no water • Gain from ingestion and metabolic water formed during aerobic respiration & dehydration synthesis reactions (2500 mL/day) • Normally loss = gain – urine, feces, sweat, breathe Tortora & Grabowski 9/e 2000 JWS 27-3 Regulation of Water Gain • Formation of metabolic water is not regulated – function of the need for ATP • Main regulator of water gain is intake regulation • Stimulators of thirst center in hypothalamus – dry mouth, osmoreceptors in hypothalamus, decreased blood volume causes drop in BP & angiotensin II • Drinking occurs – body water levels return to normal Tortora & Grabowski 9/e 2000 JWS 27-4 Dehydration Stimulates Thirst • Regulation of fluid gain is by regulation of thirst. Tortora & Grabowski 9/e 2000 JWS 27-5 Regulation of Water and Solute Loss • Elimination of excess water or solutes occurs through urination • Consumption of very salty meal demonstrates function of three hormones • Demonstrates how – “water follows salt” – excrete Na+ and water will follow and decrease blood volume Tortora & Grabowski 9/e 2000 JWS 27-6 Hormone Effects on Solutes • Angiotensin II and aldosterone promote reabsorption of Na+ and Cl- and an increase in fluid volume – stretches atrial volume and promotes release of ANP – slows release of renin & formation of angiotensin II • increases filtration rate & reduces water & Na+ reabsorption • decreases secretion of aldosterone slowing reabsorption of Na+ and Cl- in collecting ducts • ANP promotes natriuresis or the increased excretion of Na+ and Cl- which decreases blood volume Tortora & Grabowski 9/e 2000 JWS 27-7 Hormone Regulation of Water Balance • Antidiuretic hormone (ADH) from the posterior pituitary – stimulates thirst – increases permeability of principal cells of collecting ducts to assist in water reabsorption – very concentrated urine is formed • ADH secretion shuts off after the intake of water • ADH secretion is increased – large decrease in blood volume – severe dehydration and drop in blood pressure – vomiting, diarrhea, heavy sweating or burns Tortora & Grabowski 9/e 2000 JWS 27-8 Movement of Water • Intracellular and interstitial fluids normally have the same osmolarity, so cells neither swell nor shrink • Swollen cells of water intoxication because Na+ concentration of plasma falls below normal – drink plain water faster than kidneys can excrete it – replace water lost from diarrhea or vomiting with plain water – may cause convulsions, coma & death unless oral rehydration includes small amount salt in water intake Tortora & Grabowski 9/e 2000 JWS 27-9 Enemas and Fluid Balance • Introduction of a solution into the bowel to stimulate activity and evacuate feces • Increase risk of fluid & electrolyte imbalance unless isotonic solution is used Tortora & Grabowski 9/e 2000 JWS 27-10 Concentrations of Electrolytes • Functions of electrolytes – – – – control osmosis between fluid compartments help maintain acid-base balance carry electric current cofactors needed for enzymatic activity • Concentration expressed in mEq/liter or milliequivalents per liter for plasma, interstitial fluid and intracellular fluid Tortora & Grabowski 9/e 2000 JWS 27-11 Comparison Between Fluid Components • Plasma contains many proteins, but interstitial fluid does not – producing blood colloid osmotic pressure • Extracellular fluid contains Na+ and Cl• Intracellular fluid contains K+ and phosphates (HPO4 -2) Tortora & Grabowski 9/e 2000 JWS 27-12 Sodium • Most abundant extracellular ion – accounts for 1/2 of osmolarity of ECF • Average daily intake exceeds normal requirements • Hormonal controls – aldosterone causes increased reabsorption Na+ – ADH release ceases if Na+ levels too low--dilute urine lost until Na+ levels rise – ANP increases Na+ and water excretion if Na+ levels too high Tortora & Grabowski 9/e 2000 JWS 27-13 Edema, Hypovolemia and Na+ Imbalance • Sodium retention causes water retention – edema is abnormal accumulation of interstitial fluid • Causes of sodium retention – renal failure – hyperaldosterone • Excessive loss of sodium causes excessive loss of water (low blood volume) – due to inadequate secretion of aldosterone – too many diuretics Tortora & Grabowski 9/e 2000 JWS 27-14 Chloride • Most prevalent extracellular anion • Moves easily between compartments due to Clleakage channels • Helps balance anions in different compartments • Regulation – passively follows Na+ so it is regulated indirectly by aldosterone levels – ADH helps regulate Cl- in body fluids because it controls water loss in urine • Chloride shift & hydrochloric acid of gastric juice Tortora & Grabowski 9/e 2000 JWS 27-15 Potassium • Most abundant cation in intracellular fluid • Helps establish resting membrane potential & repolarize nerve & muscle tissue • Exchanged for H+ to help regulate pH in intracellular fluid • Control is mainly by aldosterone which stimulates principal cells to increase K+ secretion into the urine – abnormal plasma K+ levels adversely affect cardiac and neuromuscular function Tortora & Grabowski 9/e 2000 JWS 27-16 Bicarbonate • Common extracellular anion • Major buffer in plasma • Concentration increases as blood flows through systemic capillaries due to CO2 released from metabolically active cells • Concentration decreases as blood flows through pulmonary capillaries and CO2 is exhaled • Kidneys are main regulator of plasma levels – intercalated cells form more if levels are too low – excrete excess in the urine Tortora & Grabowski 9/e 2000 JWS 27-17 Calcium • Most abundant mineral in body (skeleton & teeth) • Abundant extracellular cation in body fluids • Important role in blood clotting, neurotransmitter release, muscle tone & nerve and muscle function • Regulated by parathyroid hormone – stimulates osteoclasts to release calcium from bone – increases production of calcitriol (Ca+2 absorption from GI tract and reabsorption from glomerular filtrate) Tortora & Grabowski 9/e 2000 JWS 27-18 Phosphate • Present as calcium phosphate in bones and teeth, and in phospholipids, ATP, DNA and RNA • HPO4 -2 is important intracellular anion and acts as buffer of H+ in body fluids and in urine – mono and dihydrogen phosphate act as buffers in the blood • Plasma levels are regulated by parathyroid hormone & calcitriol – resorption of bone releases phosphate – in the kidney, PTH increase phosphate excretion – calcitriol increases GI absorption of phosphate Tortora & Grabowski 9/e 2000 JWS 27-19 Magnesium • Found in bone matrix and as ions in body fluids – intracellular cofactor for metabolic enzymes, heart, muscle & nerve function • Urinary excretion increased in hypercalcemia, hypermagnesemia, increased extracellular fluid volume, decreases in parathyroid hormone and acidosis Tortora & Grabowski 9/e 2000 JWS 27-20 Acid-Base Balance • Homeostasis of H+ concentration is vital – proteins 3-D structure sensitive to pH changes – normal plasma pH must be maintained between 7.35 - 7.45 – diet high in proteins tends to acidify the blood • 3 major mechanisms to regulate pH – buffer system – exhalation of CO2 (respiratory system) – kidney excretion of H+ (urinary system) Tortora & Grabowski 9/e 2000 JWS 27-21 Actions of Buffer Systems • Prevent rapid, drastic changes in pH • Change either strong acid or base into weaker one • Work in fractions of a second • Found in fluids of the body • 3 principal buffer systems – protein buffer system – carbonic acid-bicarbonate buffer system – phosphate buffer system Tortora & Grabowski 9/e 2000 JWS 27-22 Protein Buffer System • Abundant in intracellular fluids & in plasma – hemoglobin very good at buffering H+ in RBCs – albumin is main plasma protein buffer • Amino acids contains at least one carboxyl group (-COOH) and at least one amino group (-NH2) – carboxyl group acts like an acid & releases H+ – amino group acts like a base & combines with H+ – some side chains can buffer H+ • Hemoglobin acts as a buffer in blood by picking up CO2 or H+ Tortora & Grabowski 9/e 2000 JWS 27-23 Carbonic Acid-Bicarbonate Buffer System • Acts as extracellular & intracellular buffer system – bicarbonate ion (HCO3-) can act as a weak base • holds excess H+ – carbonic acid (H2CO3) can act as weak acid • dissociates into H+ ions • At a pH of 7.4, bicarbonate ion concentration is about 20 times that of carbonic acid • Can not protect against pH changes due to respiratory problems Tortora & Grabowski 9/e 2000 JWS 27-24 Phosphate Buffer System • Most important intracellularly, but also acts to buffer acids in the urine • Dihydrogen phosphate ion acts as a weak acid that can buffer a strong base • Monohydrogen phosphate acts a weak base by buffering the H+ released by a strong acid Tortora & Grabowski 9/e 2000 JWS 27-25 Exhalation of Carbon Dioxide • Breathing plays a role in the homeostasis of pH • pH modified by changing rate & depth of breathing – faster breathing rate, blood pH rises – slow breathing rate, blood pH drops • H+ detected by chemoreceptors in medulla oblongata, carotid & aortic bodies • Respiratory centers inhibited or stimulated by changes is pH Tortora & Grabowski 9/e 2000 JWS 27-26 Kidney Excretion of H+ • Metabolic reactions produce 1mEq/liter of nonvolatile acid for every kilogram of body weight • Excretion of H+ in the urine is only way to eliminate huge excess • Kidneys synthesize new bicarbonate and save filtered bicarbonate • Renal failure can cause death rapidly due to its role in pH balance Tortora & Grabowski 9/e 2000 JWS 27-27 Acid-Base Imbalances • Acidosis---blood pH below 7.35 • Alkalosis---blood pH above 7.45 • Compensation is an attempt to correct the problem – respiratory compensation – renal compensation • Acidosis causes depression of CNS---coma • Alkalosis causes excitability of nervous tissue---spasms, convulsions & death Tortora & Grabowski 9/e 2000 JWS 27-28 Summary of Causes • Respiratory acidosis & alkalosis are disorders involving changes in partial pressure of CO2 in blood • Metabolic acidosis & alkalosis are disorders due to changes in bicarbonate ion concentration in blood Tortora & Grabowski 9/e 2000 JWS 27-29 Respiratory Acidosis • Cause is elevation of pCO2 of blood • Due to lack of removal of CO2 from blood – emphysema, pulmonary edema, injury to the brainstem & respiratory centers • Treatment – IV administration of bicarbonate (HCO3-) – ventilation therapy to increase exhalation of CO2 Tortora & Grabowski 9/e 2000 JWS 27-30 Respiratory Alkalosis • Arterial blood pCO2 is too low • Hyperventilation caused by high altitude, pulmonary disease, stroke, anxiety, aspirin overdose • Renal compensation involves decrease in excretion of H+ and increase reabsorption of bicarbonate • Treatment – breathe into a paper bag Tortora & Grabowski 9/e 2000 JWS 27-31 Metabolic Acidosis • Blood bicarbonate ion concentration too low – loss of ion through diarrhea or kidney dysfunction – accumulation of acid (ketosis with dieting/diabetes) – kidney failing to remove H+ from protein metabolism • Respiratory compensation by hyperventilation • Treatment – IV administration of sodium bicarbonate – correct the cause Tortora & Grabowski 9/e 2000 JWS 27-32 Metabolic Alkalosis • Blood bicarbonate levels are too high • Cause is nonrespiratory loss of acid – vomiting, gastric suctioning, use of diuretics, dehydration, excessive intake of alkaline drugs • Respiratory compensation is hypoventilation • Treatment – fluid and electrolyte therapy – correct the cause Tortora & Grabowski 9/e 2000 JWS 27-33 Diagnosis of Acid-Base Imbalances • Evaluate – systemic arterial blood pH – concentration of bicarbonate (too low or too high) – PCO2 (too low or too high) • Solutions – if problem is respiratory, the pCO2 will not be normal – if problem is metabolic, the bicarbonate level will not be normal Tortora & Grabowski 9/e 2000 JWS 27-34 Homeostasis in Infants • • • • • More body water in ECF so more easily disrupted Rate of fluid intake/output is 7X higher Higher metabolic rate produces more metabolic wastes Kidneys can not concentrate urine nor remove excess H+ Surface area to volume ratio is greater so lose more water through skin • Higher breathing rate increase water loss from lungs • Higher K+ and Cl- concentrations than adults Tortora & Grabowski 9/e 2000 JWS 27-35 Impaired Homeostasis in the Elderly • Decreased volume of intracellular fluid – inadequate fluid intake • Decreased total body K+ due to loss of muscle tissue or potassium-depleting diuretics for treatment of hypertension or heart disease • Decreased respiratory & renal function – slowing of exhalation of CO2 – decreased blood flow & glomerular filtration rate – reduced sensitivity to ADH & impaired ability to produce dilute urine – renal tubule cells produce less ammonia to combine with H+ and excrete as NH+4 Tortora & Grabowski 9/e 2000 JWS 27-36