tetrahedral site
advertisement

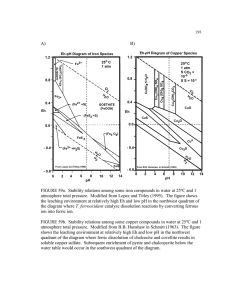
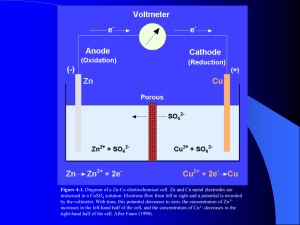
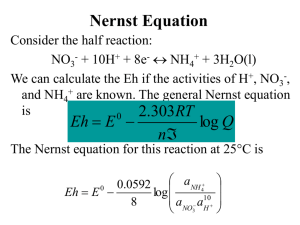
Magnetite, Fe3O4 crystallizes with the spinel structure. The large oxygen ions are close packed in a cubic arrangement and the smaller Fe ions fill in the gaps. The gaps come in two flavors: tetrahedral site: Fe ion is surrounded by four oxygens octahedral site: Fe ion is surrounded by six oxygens The tetrahedral and octahedral sites form the two magnetic sublattices, A and B respectively. The spins on the A sublattice are antiparallel to those on the B sublattice. The two crystal sites are very different and result in complex forms of exchange interactions of the iron ions between and within the two types of sites. Oxidation – Reduction diagrams…Eh vs pH diagrams Figure 4-2. Stability limits for natural waters at the earth’s surface in terms of Eh and pH at 25oC. The limits are based on partial pressures of oxygen of 1 and 10-83.1 atm. Also shown is the emf in pe units. The range of Eh and pH conditions for various natural environments is modified from Garrels and Christ (1965.) The limits of the natural Eh-pH Environment are determined by The conditions which water Breaks down to its gaesous components Ex: 4-7 goes to great lengths to demonstrate that the amount of O2 contributed to groundwater from The following redox reaction: 2H+ + ½ O2 + 2e- H2O is really really small O2 H2O H2 Water unstable Hematite Water unstable Is Fe stable In natural waters? Now we have an Eh-pH diagram that shows the conditions at which hematite and magnetite minerals are stable. We know that almost all minerals show at least some solubility…so we can add the Fe+3 and Fe+2 ions to the Eh-pH plot. The approach for doing this is Eby p. 104-106. Here are the results for Fe3+ and Fe2+. Contour lines for the activity of Fe3+ or Fe2+ All the previous can be combined into a composite Eh-pH diagram. In this case, the ions are shown to occupy regions where they are either in equilibrium or greater than the minerals. In theory, the different minerals still exist within the zones originally designated by the Eh-pH equation. In practice, they have essentially mostly dissolved into Fe2+ and/or Fe3+ ions Aqueous phase mineral phase Since we can assume that CO2 has dissolved into solution at a conc. in equilibrium, we now have some carbonate chemistry to add. Specifically, the iron carbonate mineral siderite. Lastly, include iron-sulfide complexes If this is groundwater, under what conditions is iron likely to be mobile? What happens when you pump gw from an iron-rich, but sulfur-poor aquifer, that is pH=5.8, Eh = -.050 to your bathtub exposed to the atmosphere? Martian basalt Martian blueberries Jarrahdale bauxite, Australia Arkansas bauxite Martian blueberries Continental margins Fig. 3.6 Trailing-Edge Margin Anatomy of a passive margin Continental margin Fig. 3.7 Continental slope and submarine canyons Fig. 3.8a Volcanic features of mid-ocean ridge • Hydrothermal vents – Heated subsurface seawater migrates through cracks in ocean crust • Warm-water vents <30oC or 86oF • White smokers >30oC <350oC or 662oF (white because of barium sulfide) • Black smokers > 350oC – (black because of metal sulfides; Fe, Ni, Zn) • Important for maintaining the supply of metals to the ocean Hydrothermal vents • Dissolved metals precipitate to form metal sulfide deposits • Unusual biological communities – Able to survive without sunlight – Archaeons and bacteria oxidize hydrogen sulfide gas to provide food Hydrothermal vents Fig. 3.14 Black Smoker