Diffusion-Limited Gas Exchange
advertisement

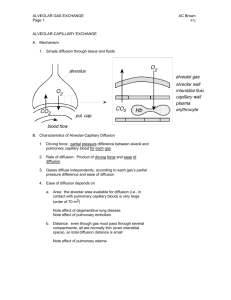
Gas Exchange and Alveolar Ventillation Objectives At the end of this session students should be able to 1.Define dead space, physiological and anatomical 2.Define partial pressure and fractional concentration as they apply to gases in air. 3. List the normal airway, alveolar, arterial, and mixed venous PO2 and PCO2 values. 3. List the normal arterial and mixed venous values for O2 saturation, [HCO3-], and pH. 4. Be able to estimate the alveolar oxygen partial pressure (PAO2) using the simplified form of the alveolar gas equation 5.Name the factors that affect diffusive transport of a gas between alveolar gas and pulmonary capillary blood. 6. Understand diffusion limited and perfusion limited gas exchange. 7.Define oxygen diffusing capacity. Dead space • Regions of the respiratory system that contain air but are not exchanging O2and CO2with blood are considered dead space. • Anatomic dead space • Airway regions that, because of inherent structure, are not capable of O2and CO2exchange with the blood. • Includes the conducting zone, which ends at the level of the terminal bronchioles. • The size of the anat VD in mL is approximately equal to a person’s weight in pounds. (Thus a 150-lb individual has an anatomic dead space of 150 mL.) • Alveolar dead space(alv VD) Refers to alveoli containing air but without blood flow in the surrounding capillaries. An example is a pulmonary embolus Physiologic dead space total dead space in the lung system (anatVD+alvVD) VENTILATION • Total Ventilation/minute volume or minute ventilation total volume of air moved in or out (usually the volume expired) of the lungs per minute. Alveolar Ventilation • Represents the air delivered to the respiratory zone(include the alveoli, alveolar sacs, alveolar ducts, and respiratory bronchioles)per breath. • Increases in the Depth of Breathing: There will be equal increases in total and alveolar ventilation per breath, since dead space volume is constant • Increases in the Rate of Breathing Increased rate causes increased ventilation of dead space and alveoli. A woman has a respiratory rate of 18, a tidal volume of 350 mL, and a dead space of 100 mL. What is her alveolar ventilation? • a. 4.0 L • b. 4.5 L • c. 5.0 L • d. 5.5 L • e. 6.0 L Gas laws • Boyle's law: when temperature is constant, pressure (P) and volume (V) are inversely related • Henry's law : that the concentration of a gas dissolved in a liquid is proportional to its partial pressure. • Henry's law is used to convert the partial pressure of gas in the liquid phase to the concentration of gas in the liquid phase (e.g., in blood). Where; • CX =Concentration of dissolved gas (mL gas/100 mL blood) and not the gas present in blood in bound form like bound to hemoglobin or plasma protein. • PX =Partial pressure of gas (mmHg) • Solubility =Solubility of gas in blood (mL gas/100 mL blood/mmHg) Dalton's law: the partial pressure of a gas in a gas mixture is the pressure that the gas would exert if it occupied the total volume of the mixture in the absence of the other components. • Partial pressure of a gas in inspired air COMPOSITION OF AIR Ambient Partial pressure- air gas Humidifie Alveolar d air air Expired air P O2 158mm 149mm 104mm 120mm P CO2 0.3mm 0.3mm 40mm 28mm P H2O 5.7mm 47mm 47mm 47mm P N2 596mm 563mm 573mm 565mm Alveolar–Blood Gas Exchange Factors affecting alveolar pCO2 1. Metabolic Rate Factors affecting alveolar pCO2 2. Alveolar Ventilation Hyperventilation • inappropriately elevated level of alveolar ventilation, and pACO2 is depressed. Hypoventilation • inappropriately depressed level of alveolar ventilation, and pACO2 is elevated. Factors affecting alveolar pO2 • The Alveolar Gas Equation • The Effect of pACO2 on pAO2 The Effect of pACO2 on pAO2 RESPIRATORY MEMBRANE 1)fluid lining alveolus 2)alveolar epithelium 3)epith basement membrane 4)interstitial space 5)Capillary basement membrane 6)capillary endothelial cell alveolar–blood gas transfer: Fick law of diffusion 1. Structural Features That Affect the Rate of Diffusion. A- area , T- thickness 2. Factors That Are Specific to Each Gas Present D- diffusion coefficient, P1-P2 = pressure diff FACTORS DETERMINING DIFFUSIONDIFFUSION COEFFICIENT • DIFFUSION COEFFICIENT=S/√MW Depends on • 1) Solubility in water 2)Molecular Weight • Though CO2 has greater molecular weight than O2 it is 20 times more soluble in water than O2. CO2 > O2> N2 Diffusing Capacity of the Respiratory Membrane • In practice, surface area, thickness, and the diffusion coefficient can be combined to yield a constant that describes the lung’s diffusing capacity (DL) for gas. Gas flow across the barrier can then be estimated from: Carbon Monoxide: A Gas that is Always Diffusion Limited • measuring the volume of carbon monoxide absorbed in a short period and dividing this by the alveolar carbon monoxide partial pressure, one can determine accurately the carbon monoxide diffusing capacity. 11. A patient inspired a gas mixture containing a trace amount of carbon monoxide and then held his breath for 10 sec. During breath holding, the alveolar PCO averaged 0.5 mm Hg and CO uptake was 10 mL/min. What is his pulmonary diffusing capacity (DLCO)? (A) 2.0 mL/min per mm Hg (B) 5.0 mL/min per mm Hg (C) 10 mL/min per mm Hg (D) 20 mL/min per mm Hg (E) 200 mL/min per mm Hg DIFFUSION-LIMITED AND PERFUSION-LIMITED GAS EXCHANGE Diffusion-limited • means that the total amount of gas transported across the alveolar-capillary is limited by the diffusion process. • In these cases, as long as the partial pressure gradient for the gas is maintained, diffusion will continue along the length of the capillary. The equilibrium is not achieved. Perfusion-limited • means that the total amount of gas transported across the alveolar/capillary membrane is limited by blood flow (i.e., perfusion) through the pulmonary capillaries. In perfusionlimited exchange, the pressure gradient is not maintained, and in this case, the only way to increase the amount of gas transported is by increasing blood flow. Equilibrium is achieved. Perfusion-Limited Gas Exchange • the transport of N2O across the alveolar/pulmonary capillary barrier – PAN2O is constant, and PaN2O is assumed to be zero at the beginning of the pulmonary capillary. – Thus, initially, there is a very large partial pressure gradient for N2O between alveolar gas and capillary blood, N2O rapidly diffuses into the pulmonary capillary. Because all of the N22O remains free in blood, all of it creates a partial pressure. Thus, the partial pressure of N2O in pulmonary capillary blood increases very rapidly and is fully equilibrated with alveolar gas in the first one fifth of the capillary. – Once equilibration occurs, there is no more partial pressure gradient and, therefore, no more driving force for diffusion. Net diffusion of N2O then ceases, although four fifths of the capillary still remain to be travelled by blood. • O2 (under normal conditions) Perfusion-limited O2 transport • In the lungs of a normal person at rest, O2 transfer from alveolar air into pulmonary capillary blood is perfusionlimited. PAO2 is constant at 100 mm Hg. • At the beginning of the capillary, PaO2 is 40 mm Hg, reflecting the composition of mixed venous blood. There is a large partial pressure gradient for O2 between alveolar air and capillary blood, which causes diffusion into the capillary. As O2 is added to pulmonary capillary blood, PaO2 increases. The gradient for diffusion is maintained initially because O2 binds to hemoglobin, which keeps the free O2 concentration in blood and the partial pressure low. Equilibration of O2 occurs about one third of the distance along the capillary, at which point PaO2 becomes equal to PAO2, and unless blood flow increases, there can be no more net diffusion of O2. Thus, under normal conditions, transport is perfusionlimited. Diffusion-limited O2 transport • In certain pathologic conditions (e.g., fibrosis) and during strenuous exercise, transfer becomes diffusion limited. • Fibrosis: – In fibrosis the alveolar wall thickens, increasing the diffusion distance for gases across the wall and decreasing DL which slows the rate of diffusion of O2 and prevents equilibration of O2 between alveolar air and pulmonary capillary blood. In these cases, the partial pressure gradient for O2 is maintained along the entire length of the capillary, converting it to a diffusion-limited process (although not as extreme as in the example of CO). – At the end of the pulmonary capillary, equilibration has not occurred between alveolar air and pulmonary capillary blood (PaO2 is less than PAO2), which will be reflected in a decreased PaO2 in systemic arterial blood and decreased PvO2 in mixed venous blood. • O2 diffusion along the length of the pulmonary capillary in normal persons and persons with fibrosis. A, at sea level and B, at high altitude. O2 transport at high altitude • Ascent to high altitude alters some aspects of the O2 equilibration process. At high altitude, barometric pressure is reduced, and with the same fraction of O2 in inspired air, the partial pressure of O2 in alveolar gas also will be reduced. • PAO2 is reduced to 50 mm Hg, compared the normal value of 100 mm Hg. Mixed venous PO2 is 25 mm Hg (as opposed to the normal value of 40 mm Hg). Therefore, at high altitude, the partial pressure gradient for O2 is greatly reduced compared with sea level. Even at the beginning of the pulmonary capillary, the gradient is only 25 mm Hg (50 mm Hg - 25 mmHg) instead of the normal gradient at sea level of 60 mm Hg (100 mm Hg - 40 mm Hg). This reduction of the partial pressure gradient means that diffusion of O2 will be reduced, equilibration will occur more slowly along the capillary, complete equilibration will be achieved at a later point along the capillary (two-thirds of the capillary length at altitude, compared with one third of the length at sea level). • The final equilibrated value for PaO2 is only 50mmHg because PAO2 is only 50 mm Hg (it is impossible for the equilibrated value to be higher than 50 mm Hg). The equilibration of O2 at high altitude is exaggerated in a person with fibrosis. Pulmonary capillary blood does not equilibrate even by the end of the capillary, resulting in values for PaO2 as low as 30 mm Hg, which will seriously hamper O2 delivery to the tissues. Examples Diffusion-Limited Gas Exchange • the transport of CO across the alveolar/pulmonary capillary barrier – net diffusion of CO into the pulmonary capillary depends on the magnitude of the partial pressure gradient, is maintained because CO is bound to hemoglobin in capillary blood. Recall that only free, dissolved gas causes a partial pressure. Thus, CO does not equilibrate by the end of the capillary. In fact, if the capillary were longer, net diffusion would continue indefinitely, or until equilibration occurred. • the transport of O2 during strenuous exercise and in pathologic conditions such as emphysema and fibrosis.