Empirical and Molecular Formula
advertisement

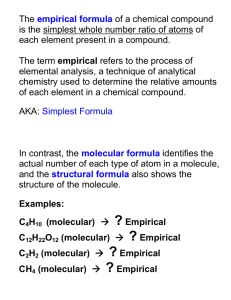
Empirical and Molecular Formula Miss Knick HAHS Empirical Formula O Is the simplest whole number ratio of the atoms in a compound Practice: Empirical or Not…is the question. CH4 P2O5 C 2H 6 P4H10 Calculating the empirical formula 1. Convert the mass of each element to moles 2. Divide each number of moles by the smallest number of moles of all the answers to #1 3. If the answers to #2 are whole numbers, these are the subscripts in the empirical formula If any of the answers are not whole numbers, covert all answers to a common fraction. Practice (skip 8 lines between each) O Ex #1: The compound that gives vinegar its sour taste is acetic acid, which contains the elements carbon, hydrogen, and oxygen. When 5.00grams of the compound is analyzed, it is found to contain 2 g of carbon, 0.336 g of hydrogen, and 2.66 g of oxygen. What is the empirical formula? O Ex. #2: A compound contains 36.5% sodium, 25.4% sulfur, and 38.1% oxygen. What is the empirical formula? Molecular Formula O The true ratio of the atoms in a compound as it exists naturally; may be the same as the empirical formula; will relate to the empirical formula in whole number ratios Calculating Molecular Formula O 1. Find the molar mass of the empirical formula. O 2. Divide the molecular formula mass by the empirical formula mass. O 3. Multiple each subscript in the empirical formula by the answer to #2, these are the subscripts for the molecular formula Mass Spectrometer 7 Tro: Chemistry: A Molecular Approach, 2/e Practice O Ex #3: The molar mass of acetic acid has been determined to be 60 g/mol. Using that information with the simplest formula calculated in Ex #1, determine the molecular formula of acetic acid.